Targeted Complement Inhibition at Synapses Prevents Microglial Synaptic Engulfment and Synapse Loss in Demyelinating Disease
- PMID: 31883839
- PMCID: PMC6996144
- DOI: 10.1016/j.immuni.2019.12.004
Targeted Complement Inhibition at Synapses Prevents Microglial Synaptic Engulfment and Synapse Loss in Demyelinating Disease
Abstract
Multiple sclerosis (MS) is a demyelinating, autoimmune disease of the central nervous system. While work has focused on myelin and axon loss in MS, less is known about mechanisms underlying synaptic changes. Using postmortem human MS tissue, a preclinical nonhuman primate model of MS, and two rodent models of demyelinating disease, we investigated synapse changes in the visual system. Similar to other neurodegenerative diseases, microglial synaptic engulfment and profound synapse loss were observed. In mice, synapse loss occurred independently of local demyelination and neuronal degeneration but coincided with gliosis and increased complement component C3, but not C1q, at synapses. Viral overexpression of the complement inhibitor Crry at C3-bound synapses decreased microglial engulfment of synapses and protected visual function. These results indicate that microglia eliminate synapses through the alternative complement cascade in demyelinating disease and identify a strategy to prevent synapse loss that may be broadly applicable to other neurodegenerative diseases. VIDEO ABSTRACT.
Keywords: complement; demyelination; engulfment; gene therapy; microglia; multiple sclerosis; neural-immune; neurodegeneration; neuroinflammation; synapse.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no conflict of interest.
Figures

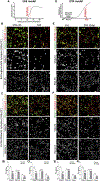

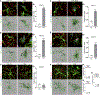

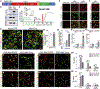

Comment in
-
That Wasn't a Complement-Too Much C3 in Demyelinating Disease.Immunity. 2020 Jan 14;52(1):11-13. doi: 10.1016/j.immuni.2019.12.014. Immunity. 2020. PMID: 31951547 Free PMC article.
References
-
- AEINEHBAND S, LINDBLOM RP, AL NIMER F, VIJAYARAGHAVAN S, SANDHOLM K, KHADEMI M, OLSSON T, NILSSON B, EKDAHL KN, DARREH-SHORI T & PIEHL F 2015. Complement component C3 and butyrylcholinesterase activity are associated with neurodegeneration and clinical disability in multiple sclerosis. PLoS One, 10, e0122048. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

