Visible-Light-Induced Alkoxyl Radicals Enable α-C(sp3)-H Bond Allylation
- PMID: 31884167
- PMCID: PMC6941871
- DOI: 10.1016/j.isci.2019.100755
Visible-Light-Induced Alkoxyl Radicals Enable α-C(sp3)-H Bond Allylation
Abstract
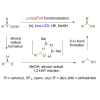
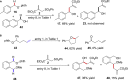
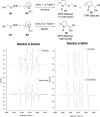
The alkoxyl radical is an essential reactive intermediate in mechanistic studies and organic synthesis with hydrogen atom transfer (HAT) reactivity. However, compared with intramolecular 1,5-HAT or intermolecular HAT of alkoxyl radicals, the intramolecular 1,2-HAT reactivity has been limited to theoretical studies and rarely synthetically utilized. Here we report the first selective 1,2-HAT of alkoxyl radicals for α-C(sp3)-H bond allylation of α-carbonyl, α-cyano, α-trifluoromethyl, and benzylic N-alkoxylphthalimides. The mechanistic probing experiments, electron paramagnetic resonance (EPR) studies, and density functional theory (DFT) calculations confirmed the 1,2-HAT reactivity of alkoxyl radicals, and the use of protic solvents lowered the activation energy by up to 10.4 kcal/mol to facilitate the α-C(sp3)-H allylation reaction.
Keywords: Organic Chemistry; Organic Reaction; Physical Organic Chemistry.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures








References
-
- Azizi S., Ulrich G., Guglielmino M., le Calve S., Hagon J.P., Harriman A., Ziessel R. Photoinduced proton transfer promoted by peripheral subunits for some Hantzsch esters. J. Phys. Chem. A. 2015;119:39–49. - PubMed
-
- Bietti M., Salamone M. Reaction pathways of alkoxyl radicals. The role of solvent effects on C–C bond fragmentation and hydrogen atom transfer reactions. Synlett. 2014;25:1803–1816.
-
- Burke S.D., Silks L.A., Strickland S.M.S. Remote functionalization and molecular modeling: observations relevant to the barton and hypoiodite reactions. Tetrahedron Lett. 1988;29:2761–2764.
-
- Buszek R.J., Sinha A., Francisco J.S. The isomerization of methoxy radical: intramolecular hydrogen atom transfer mediated through acid catalysis. J. Am. Chem. Soc. 2011;133:2013–2015. - PubMed
-
- Čeković Ž. Reactions of δ-carbon radicals generated by 1,5-hydrogen transfer to alkoxyl radicals. Tetrahedron. 2003;59:8073–8090.
LinkOut - more resources
Full Text Sources

