24-Month Phase I/II Clinical Trial of Bimatoprost Sustained-Release Implant (Bimatoprost SR) in Glaucoma Patients
- PMID: 31884564
- PMCID: PMC7007425
- DOI: 10.1007/s40265-019-01248-0
24-Month Phase I/II Clinical Trial of Bimatoprost Sustained-Release Implant (Bimatoprost SR) in Glaucoma Patients
Abstract
Objective: The objective of this study was to evaluate the safety and intraocular pressure (IOP)-lowering effects over 24 months of biodegradable bimatoprost sustained-release implant (Bimatoprost SR) administration versus topical bimatoprost 0.03% in patients with open-angle glaucoma (OAG).
Methods: This was a phase I/II, prospective, 24-month, dose-ranging, paired-eye controlled clinical trial. At baseline following washout, adult patients with OAG (N = 75) received Bimatoprost SR (6, 10, 15, or 20 µg) intracamerally in the study eye; the fellow eye received topical bimatoprost 0.03% once daily. Rescue topical IOP-lowering medication or single repeat administration with implant was permitted. The primary endpoint was IOP change from baseline. Safety measures included adverse events (AEs).
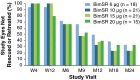
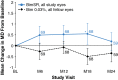
Results: At month 24, mean IOP reduction from baseline was 7.5, 7.3, 7.3, and 8.9 mmHg in eyes treated with Bimatoprost SR 6, 10, 15, and 20 µg, respectively, versus 8.2 mmHg in pooled fellow eyes; 68, 40, and 28% of pooled study eyes had not been rescued/retreated at months 6, 12, and 24, respectively. AEs in study eyes that occurred ≤ 2 days post-procedure typically were transient. After 2 days post-procedure, overall AE incidence was similar between study and fellow eyes, with some events typically associated with topical prostaglandin analogs having lower incidence in study eyes.
Conclusions: Bimatoprost SR showed favorable efficacy and safety profiles up to 24 months, with all evaluated dose strengths demonstrating overall IOP-reducing effects comparable to those of topical bimatoprost. Targeted and sustained delivery of bimatoprost resulted in protracted IOP lowering, suggesting that Bimatoprost SR may represent a transformational new approach to glaucoma therapy. Clinicaltrials.gov identifier: NCT01157364.
Conflict of interest statement
E. Randy Craven is a consultant for Aerie, Alcon, Allergan, Ivantis, Santen, and WL Gore and has received grant support from Allergan. Thomas Walters is a consultant for Allergan, Nicox, Ocular Therapeutix, and Sun. William C. Christie is a consultant for Allergan and Johnson & Johnson. Douglas G. Day is a consultant for Allergan. Richard A. Lewis is a consultant for Aerie, Alcon, Allergan, Aquesys, AVS, Envisia, Glaukos, Ivantis, Oculeve, PolyActiva, ViSci, and Zeiss. Margot L. Goodkin, Michelle Chen, Veronica Wangsadipura, Michael R. Robinson, and Marina Bejanian are Allergan employees.
Figures



References
-
- American Academy of Ophthalmology. Preferred practice pattern in primary open-angle glaucoma; 2016. https://www.aao.org/preferred-practice-pattern/primary-open-angle-glauco.... Accessed Sep 17, 2019.
-
- European Glaucoma Society. Terminology and guidelines for glaucoma (4th edition); 2014. http://www.eugs.org/eng/EGS_guidelines4.asp. Accessed Oct 18, 2016.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

