TGFB-INHB/activin signaling regulates age-dependent autophagy and cardiac health through inhibition of MTORC2
- PMID: 31884871
- PMCID: PMC8386626
- DOI: 10.1080/15548627.2019.1704117
TGFB-INHB/activin signaling regulates age-dependent autophagy and cardiac health through inhibition of MTORC2
Abstract
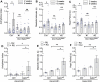
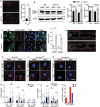
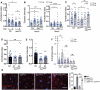
Age-related impairment of macroautophagy/autophagy and loss of cardiac tissue homeostasis contribute significantly to cardiovascular diseases later in life. MTOR (mechanistic target of rapamycin kinase) signaling is the most well-known regulator of autophagy, cellular homeostasis, and longevity. The MTOR signaling consists of two structurally and functionally distinct multiprotein complexes, MTORC1 and MTORC2. While MTORC1 is well characterized but the role of MTORC2 in aging and autophagy remains poorly understood. Here we identified TGFB-INHB/activin signaling as a novel upstream regulator of MTORC2 to control autophagy and cardiac health during aging. Using Drosophila heart as a model system, we show that cardiac-specific knockdown of TGFB-INHB/activin-like protein daw induces autophagy and alleviates age-related heart dysfunction, including cardiac arrhythmias and bradycardia. Interestingly, the downregulation of daw activates TORC2 signaling to regulate cardiac autophagy. Activation of TORC2 alone through overexpressing its subunit protein rictor promotes autophagic flux and preserves cardiac function with aging. In contrast, activation of TORC1 does not block autophagy induction in daw knockdown flies. Lastly, either daw knockdown or rictor overexpression in fly hearts prolongs lifespan, suggesting that manipulation of these pathways in the heart has systemic effects on longevity control. Thus, our studies discover the TGFB-INHB/activin-mediated inhibition of TORC2 as a novel mechanism for age-dependent decreases in autophagic activity and cardiac health. Abbreviations: AI: arrhythmia index; BafA1: bafilomycin A1; BMP: bone morphogenetic protein; CQ: chloroquine; CVD: cardiovascular diseases; DI: diastolic interval; ER: endoplasmic reticulum; HP: heart period; HR: heart rate; MTOR: mechanistic target of rapamycin kinase; NGS: normal goat serum; PBST: PBS with 0.1% Triton X-100; PDPK1: 3-phosphoinositide dependent protein kinase 1; RICTOR: RPTOR independent companion of MTOR complex 2; ROI: region of interest; ROUT: robust regression and outlier removal; ROS: reactive oxygen species; R-SMAD: receptor-activated SMAD; SI: systolic interval; SOHA: semi-automatic optical heartbeat analysis; TGFB: transformation growth factor beta; TSC1: TSC complex subunit 1.
Keywords: Atg8a; INHB/activin ligand; TOR complex 2; autophagic flux; dawdle; semi-automatic optical heartbeat analysis (SOHA).
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
