Real world hybrid closed-loop discontinuation: Predictors and perceptions of youth discontinuing the 670G system in the first 6 months
- PMID: 31885123
- PMCID: PMC7204392
- DOI: 10.1111/pedi.12971
Real world hybrid closed-loop discontinuation: Predictors and perceptions of youth discontinuing the 670G system in the first 6 months
Abstract
Objective: To describe predictors of hybrid closed loop (HCL) discontinuation and perceived barriers to use in youth with type 1 diabetes.
Subjects: Youth with type 1 diabetes (eligible age 2-25 y; recruited age 8-25 y) who initiated the Minimed 670G HCL system were followed prospectively for 6 mo in an observational study.
Research design and methods: Demographic, glycemic (time-in-range, HbA1c), and psychosocial variables [Hypoglycemia Fear Survey (HFS); Problem Areas in Diabetes (PAID)] were collected for all participants. Participants who discontinued HCL (<10% HCL use at clinical visit) completed a questionnaire on perceived barriers to HCL use.
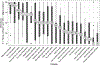
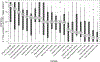
Results: Ninety-two youth (15.7 ± 3.6 y, HbA1c 8.8 ± 1.3%, 50% female) initiated HCL, and 28 (30%) discontinued HCL, with the majority (64%) discontinuing between 3 and 6 mo after HCL start. Baseline HbA1c predicted discontinuation (P = .026) with the odds of discontinuing 2.7 times higher (95% CI: 1.123, 6.283) for each 1% increase in baseline HbA1c. Youth who discontinued HCL rated difficulty with calibrations, number of alarms, and too much time needed to make the system work as the most problematic aspects of HCL. Qualitatively derived themes included technological difficulties (error alerts, not working correctly), too much work (calibrations, fingersticks), alarms, disappointment in glycemic control, and expense (cited by parents).
Conclusions: Youth with higher HbA1c are at greater risk for discontinuing HCL than youth with lower HbA1c, and should be the target of new interventions to support device use. The primary reasons for discontinuing HCL relate to the workload required to use HCL.
Keywords: artificial pancreas; continuous glucose monitor; hybrid closed loop; insulin pump; pediatrics; type 1 diabetes.
© 2019 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures
References
-
- EURODIAB ACE Study Group. Variation and trends in incidence of childhood diabetes in Europe, EURODIAB ACE Study Group. Lancet. 2000;355:873–876. - PubMed
-
- Harjutsalo V, Sjoberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371: 1777–1782. - PubMed
-
- American Diabetes Association. Glycemic targets: standards of medical care in diabetes 2019. Diabetes Care. 2019;42:S61–S70. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical