Increased Soluble Epoxide Hydrolase in Human Gestational Tissues from Pregnancies Complicated by Acute Chorioamnionitis
- PMID: 31885501
- PMCID: PMC6915158
- DOI: 10.1155/2019/8687120
Increased Soluble Epoxide Hydrolase in Human Gestational Tissues from Pregnancies Complicated by Acute Chorioamnionitis
Abstract
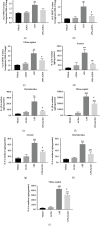
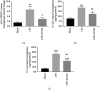
Chorioamnionitis (CAM) is primarily a polymicrobial bacterial infection involving chorionic and amniotic membranes that is associated with increased risk of preterm delivery. Epoxyeicosatrienoic acids (EETs) are eicosanoids generated from arachidonic acid by cytochrome P450 enzymes and further metabolized mainly by soluble epoxide hydrolase (sEH) to produce dihydroxyeicosatrienoic acids (DHETs). As a consequence of this metabolism of EETs, sEH reportedly exacerbates several disease states; however, its role in CAM remains unclear. The objectives of this study were to (1) determine the localization of sEH and compare the changes it undergoes in the gestational tissues (placentas and fetal membranes) of women with normal-term pregnancies and those with pregnancies complicated by acute CAM; (2) study the effects of lipopolysaccharide (LPS) on the expression of sEH in the human gestational tissues; and (3) investigate the effect of 12-(3-adamantan-1-yl-ureido)-dodecanoic acid (AUDA), a specific sEH inhibitor, on LPS-induced changes in 14,15-DHET and cytokines such as interleukin- (IL-) 1β and IL-6 in human gestational tissues in vitro and in pregnant mice. We found that women with pregnancies complicated by acute CAM had higher levels of sEH mRNA and protein in fetal membranes and villous tissues compared to those in women with normal-term pregnancies without CAM. Furthermore, fetal membrane and villous explants treated with LPS had higher tissue levels of sEH mRNA and protein and 14,15-DHET than those present in the vehicle controls, while the administration of AUDA in the media attenuated the LPS-induced production of 14,15-DHET in tissue homogenates and IL-1β and IL-6 in the media of explant cultures. Administration of AUDA also reduced the LPS-induced changes of 14,15-DHET, IL-1β, and IL-6 in the placentas of pregnant mice. Together, these results suggest that sEH participates in the inflammatory changes in human gestational tissues in pregnancies complicated by acute CAM.
Copyright © 2019 Tai-Ho Hung et al.
Conflict of interest statement
All the authors declare that they have no conflicts of interest.
Figures





References
-
- Mueller-Heubach E., Rubinstein D. N., Schwarz S. S. Histologic chorioamnionitis and preterm delivery in different patient populations. Obstetrics and Gynecology. 1990;75(4):622–626. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

