Optimizing Laser Capture Microdissection Protocol for Isolating Zone-Specific Cell Populations from Mandibular Condylar Cartilage
- PMID: 31885587
- PMCID: PMC6914897
- DOI: 10.1155/2019/5427326
Optimizing Laser Capture Microdissection Protocol for Isolating Zone-Specific Cell Populations from Mandibular Condylar Cartilage
Abstract
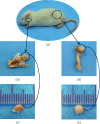
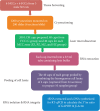
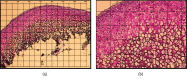
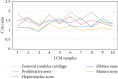
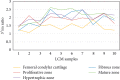
Mandibular condylar cartilage (MCC) is a multizonal heterogeneous fibrocartilage consisting of fibrous (FZ), proliferative (PZ), mature (MZ), and hypertrophic (HZ) zones. Gross sampling of the whole tissue may conceal some important information and compromise the validity of the molecular analysis. Laser capture microdissection (LCM) technology allows isolating zonal (homogenous) cell populations and consequently generating more accurate molecular and genetic data, but the challenges during tissue preparation and microdissection procedures are to obtain acceptable tissue section morphology that allows histological identification of the desirable cell type and to minimize RNA degradation. Therefore, our aim is to optimize an LCM protocol for isolating four homogenous zone-specific cell populations from their respective MCC zones while preserving the quality of RNA recovered. MCC and FCC (femoral condylar cartilage) specimens were harvested from 5-week-old Sprague-Dawley male rats. Formalin-fixed and frozen unfixed tissue sections were prepared and compared histologically. Additional specimens were microdissected to prepare LCM samples from FCC and each MCC zone individually. Then, to evaluate LCM-RNA integrity, 3'/m ratios of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and beta-actin (β-Actin) using quantitative reverse transcription-polymerase chain reaction (qRT-PCR) were calculated. Both fixed and unfixed tissue sections allowed reliable identification of MCC zones. The improved morphology of the frozen sections of our protocol has extended the range of cell types to be isolated. Under the empirically set LCM parameters, four homogeneous cell populations were efficiently isolated from their respective zones. The 3'/m ratio means of GAPDH and β-Actin ranged between 1.11-1.56 and 1.41-2.12, respectively. These values are in line with the reported quality control requirements. The present study shows that the optimized LCM protocol could allow isolation of four homogenous zone-specific cell populations from MCC, meanwhile preserving RNA integrity to meet the high quality requirements for subsequent molecular analyses. Thereby, accurate molecular and genetic data could be generated.
Copyright © 2019 Aisha M. Basudan and Yanqi Yang.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Figures







Similar articles
-
Implications of zonal architecture on differential gene expression profiling and altered pathway expressions in mandibular condylar cartilage.Sci Rep. 2021 Aug 19;11(1):16915. doi: 10.1038/s41598-021-96071-7. Sci Rep. 2021. PMID: 34413358 Free PMC article.
-
Effects of fixation on RNA extraction and amplification from laser capture microdissected tissue.Mol Carcinog. 1999 Jun;25(2):86-91. Mol Carcinog. 1999. PMID: 10365909
-
Immuno-LCM: laser capture microdissection of immunostained frozen sections for mRNA analysis.Am J Pathol. 1999 Jan;154(1):61-6. doi: 10.1016/S0002-9440(10)65251-0. Am J Pathol. 1999. PMID: 9916919 Free PMC article.
-
Laser capture microdissection in pathology.J Clin Pathol. 2000 Sep;53(9):666-72. doi: 10.1136/jcp.53.9.666. J Clin Pathol. 2000. PMID: 11041055 Free PMC article. Review.
-
Laser capture microdissection: methodical aspects and applications with emphasis on immuno-laser capture microdissection.Pathobiology. 2000;68(4-5):209-14. doi: 10.1159/000055925. Pathobiology. 2000. PMID: 11279348 Review.
Cited by
-
Implications of zonal architecture on differential gene expression profiling and altered pathway expressions in mandibular condylar cartilage.Sci Rep. 2021 Aug 19;11(1):16915. doi: 10.1038/s41598-021-96071-7. Sci Rep. 2021. PMID: 34413358 Free PMC article.
-
Sulfur-species in Zinc-specific Condylar Zones of a Rat Temporomandibular Joint.bioRxiv [Preprint]. 2024 Nov 14:2024.11.11.623079. doi: 10.1101/2024.11.11.623079. bioRxiv. 2024. PMID: 39605645 Free PMC article. Preprint.
References
LinkOut - more resources
Full Text Sources
Research Materials

