Strategy for the Generation of Engineered Bone Constructs Based on Umbilical Cord Mesenchymal Stromal Cells Expanded with Human Platelet Lysate
- PMID: 31885622
- PMCID: PMC6914958
- DOI: 10.1155/2019/7198215
Strategy for the Generation of Engineered Bone Constructs Based on Umbilical Cord Mesenchymal Stromal Cells Expanded with Human Platelet Lysate
Abstract
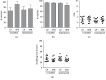
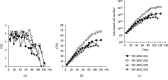
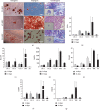
Umbilical cord mesenchymal stromal cells (UC-MSC) are promising candidates for cell therapy due to their potent multilineage differentiation, enhanced self-renewal capacity, and immediate availability for clinical use. Clinical experience has demonstrated satisfactory biosafety profiles and feasibility of UC-MSC application in the allogeneic setting. However, the use of UC-MSC for bone regeneration has not been fully established. A major challenge in the generation of successful therapeutic strategies for bone engineering lies on the combination of highly functional proosteogenic MSC populations and bioactive matrix scaffolds. To address that, in this study we proposed a new approach for the generation of bone-like constructs based on UC-MSC expanded in human platelet lysate (hPL) and evaluated its potential to induce bone structures in vivo. In order to obtain UC-MSC for potential clinical use, we first assessed parameters such as the isolation method, growth supplementation, microbiological monitoring, and cryopreservation and performed full characterization of the cell product including phenotype, growth performance, tree-lineage differentiation, and gene expression. Finally, we evaluated bone-like constructs based on the combination of stimulated UC-MSC and collagen microbeads for in vivo bone formation. UC-MSC were successfully cultured from 100% of processed UC donors, and efficient cell derivation was observed at day 14 ± 3 by the explant method. UC-MSC maintained mesenchymal cell morphology, phenotype, high cell growth performance, and probed multipotent differentiation capacity. No striking variations between donors were recorded. As expected, UC-MSC showed tree-lineage differentiation and gene expression profiles similar to bone marrow- and adipose-derived MSC. Importantly, upon osteogenic and endothelial induction, UC-MSC displayed strong proangiogenic and bone formation features. The combination of hPL-expanded MSC and collagen microbeads led to bone/vessel formation following implantation into an immune competent mouse model. Collectively, we developed a high-performance UC-MSC-based cell manufacturing bioprocess that fulfills the requirements for human application and triggers the potency and effectivity of cell-engineered scaffolds for bone regeneration.
Copyright © 2019 Ingrid Silva-Cote et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures




Similar articles
-
Minimally manipulated whole human umbilical cord is a rich source of clinical-grade human mesenchymal stromal cells expanded in human platelet lysate.Cytotherapy. 2011 Aug;13(7):786-801. doi: 10.3109/14653249.2011.563294. Epub 2011 Mar 18. Cytotherapy. 2011. PMID: 21417678
-
Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials.Haematologica. 2006 Aug;91(8):1017-26. Epub 2006 Jul 25. Haematologica. 2006. PMID: 16870554
-
The osteogenic differentiation of adult bone marrow and perinatal umbilical mesenchymal stem cells and matrix remodelling in three-dimensional collagen scaffolds.Biomaterials. 2010 Jan;31(3):467-80. doi: 10.1016/j.biomaterials.2009.09.059. Epub 2009 Oct 7. Biomaterials. 2010. PMID: 19815272
-
Use of platelet lysate for bone regeneration - are we ready for clinical translation?World J Stem Cells. 2016 Feb 26;8(2):47-55. doi: 10.4252/wjsc.v8.i2.47. World J Stem Cells. 2016. PMID: 26981170 Free PMC article. Review.
-
Umbilical cord fibroblasts: Could they be considered as mesenchymal stem cells?World J Stem Cells. 2014 Jul 26;6(3):367-70. doi: 10.4252/wjsc.v6.i3.367. World J Stem Cells. 2014. PMID: 25126385 Free PMC article. Review.
Cited by
-
Mesenchymal Stem/Progenitor Cells: The Prospect of Human Clinical Translation.Stem Cells Int. 2020 Aug 11;2020:8837654. doi: 10.1155/2020/8837654. eCollection 2020. Stem Cells Int. 2020. PMID: 33953753 Free PMC article. Review.
-
Human Platelet Lysate Supports Efficient Expansion and Stability of Wharton's Jelly Mesenchymal Stromal Cells via Active Uptake and Release of Soluble Regenerative Factors.Int J Mol Sci. 2020 Aug 31;21(17):6284. doi: 10.3390/ijms21176284. Int J Mol Sci. 2020. PMID: 32877987 Free PMC article.
-
Large and small extracellular vesicles from Wharton's jelly MSCs: Biophysics, function, and strategies to improve immunomodulation.Mol Ther Methods Clin Dev. 2024 Oct 9;32(4):101353. doi: 10.1016/j.omtm.2024.101353. eCollection 2024 Dec 12. Mol Ther Methods Clin Dev. 2024. PMID: 39512906 Free PMC article.
-
Bioengineered skin constructs based on mesenchymal stromal cells and acellular dermal matrix exposed to inflammatory microenvironment releasing growth factors involved in skin repair.Stem Cell Res Ther. 2023 Oct 26;14(1):306. doi: 10.1186/s13287-023-03535-w. Stem Cell Res Ther. 2023. PMID: 37880776 Free PMC article.
-
Integrated Analysis of Transcriptome and Secretome From Umbilical Cord Mesenchymal Stromal Cells Reveal New Mechanisms for the Modulation of Inflammation and Immune Activation.Front Immunol. 2020 Sep 30;11:575488. doi: 10.3389/fimmu.2020.575488. eCollection 2020. Front Immunol. 2020. PMID: 33117373 Free PMC article.
References
-
- Carlsson P.-O., Svahn M. Wharton’s jelly derived allogeneic mesenchymal stromal cells for treatment of type 1 diabetes: study protocol for a double-blinded, randomized, parallel, placebo-controlled trial. Clinical Trials in Degenerative Diseases. 2018;3(2):32–37. doi: 10.4103/2542-3975.235141. - DOI
-
- Chen Y.-S. Mesenchymal stem cell: considerations for manufacturing and clinical trials on cell therapy product. International Journal of Stem cell Research & Therapy. 2016;3(1) doi: 10.23937/2469-570x/1410029. - DOI
-
- Galipeau J., Krampera M., Barrett J., et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18(2):151–159. doi: 10.1016/j.jcyt.2015.11.008. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

