Heme Oxygenases: Cellular Multifunctional and Protective Molecules against UV-Induced Oxidative Stress
- PMID: 31885801
- PMCID: PMC6907065
- DOI: 10.1155/2019/5416728
Heme Oxygenases: Cellular Multifunctional and Protective Molecules against UV-Induced Oxidative Stress
Abstract
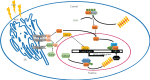
Ultraviolet (UV) irradiation can be considered as a double-edged sword: not only is it a crucial environmental factor that can cause skin-related disorders but it can also be used for phototherapy of skin diseases. Inducible heme oxygenase-1 (HO-1) in response to a variety of stimuli, including UV exposure, is vital to maintain cell homeostasis. Heme oxygenase-2 (HO-2), another member of the heme oxygenase family, is constitutively expressed. In this review, we discuss how heme oxygenase (HO), a vital rate-limiting enzyme, participates in heme catabolism and cytoprotection. Phylogenetic analysis showed that there may exist a functional differentiation between HO-1 and HO-2 during evolution. Furthermore, depending on functions in immunomodulation and antioxidation, HO-1 participates in disease progression, especially in pathogenesis of skin diseases, such as vitiligo and psoriasis. To further investigate the particular role of HO-1 in diseases, we summarized the profile of the HO enzyme system and its related signaling pathways, such as Nrf2 and endoplasmic reticulum crucial signaling, both known to regulate HO-1 expression. Furthermore, we report on a C-terminal truncation of HO-1, which is generally considered as a signal molecule. Also, a newly identified alternative splice isoform of HO-1 not only provides us a novel perspective on comprehensive HO-1 alternative splicing but also offers us a basis to clarify the relationship between HO-1 transcripts and oxidative diseases. To conclude, the HO system is not only involved in heme catabolism but also involved in biological processes related to the pathogenesis of certain diseases, even though the mechanism of disease progression still remains sketchy. Further understanding the role of the HO system and its relationship to UV is helpful for revealing the HO-related signaling networks and the pathogenesis of many diseases.
Copyright © 2019 ShiDa Chen et al.
Conflict of interest statement
The authors declared that there are no potential conflicts of interest.
Figures

References
-
- Dennery P. A. Current Topics in Cellular Regulation. Vol. 36. Elsevier; 2001. Regulation and role of heme oxygenase in oxidative injury; pp. 181–199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

