Removal of Fluoride from Drinking Water by Sorption Using Diatomite Modified with Aluminum Hydroxide
- PMID: 31886021
- PMCID: PMC6914935
- DOI: 10.1155/2019/4831926
Removal of Fluoride from Drinking Water by Sorption Using Diatomite Modified with Aluminum Hydroxide
Abstract
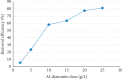
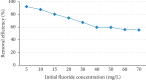
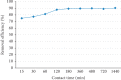
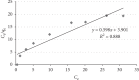
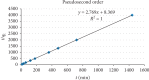
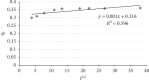
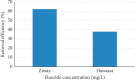
Exposure to fluoride beyond the recommended level for longer duration causes both dental and skeletal fluorosis. Thus, the development of cost-effective, locally available, and environmentally benign adsorbents for fluoride removal from contaminated water sources is absolutely required. In the present study, diatomaceous earth (diatomite) locally available in Ethiopia, modified by treating it with an aluminum hydroxide solution, was used as an adsorbent for fluoride removal from aqueous solutions. Adsorption experiments were carried out by using batch contact method. The adsorbent was characterized using FT-IR spectroscopy. Effects of different parameters affecting efficiency of fluoride removal such as adsorbent dose, contact time, initial fluoride concentration, and pH were investigated and optimized. The optimum adsorbent dose, contact time, initial fluoride concentration, and pH values were 25 g/L, 180 min, 10 mg/L, and 6.7, respectively. The performance of the adsorbent was also tested under optimum conditions using groundwater samples taken from Hawassa and Ziway. Langmuir and Freundlich isotherm models were applied to describe the equilibrium data. Compared to Langmuir isotherm (R 2 = 0.888), the Freundlich isotherm (R 2 = 0.985) model was better fitted to describe the adsorption characteristics of fluoride on Al-diatomite. The Langmuir maximum adsorption capacity was 1.67 mg/g. The pseudosecond-order model was found to be more suitable than the pseudofirst-order to describe the adsorption kinetics. The low correlation coefficient value of R 2 = 0.596 for the intraparticle diffusion model indicates that the intraparticle diffusion model does not apply to the present studied adsorption system. The maximum fluoride removal was observed to be 89.4% under the optimum conditions which indicated that aluminum hydroxide-modified diatomite can be used as efficient, cheap, and ecofriendly adsorbents for the removal of fluoride from contaminated water.
Copyright © 2019 Tesfaye Akafu et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest regarding the publication of this paper.
Figures




References
-
- Grim J., Bessarobov D., Sanderson R. Review of electro-assisted methods for water purification. Desalination. 1998;115:285–294.
-
- Tewari A., Dubey A. Defluoridation of drinking water: efficacy and need. Journal of Chemical and Pharmaceutical Research. 2009;1(1):31–37.
-
- Viswanathan G., Gopalakrishnan S., Siva Ilango S. Assessment of water contribution on total fluoride intake of various age groups of people in fluoride endemic and non-endemic areas of Dindigul District, Tamil Nadu, South India. Water Research. 2010;44(20):6186–6200. doi: 10.1016/j.watres.2010.07.041. - DOI - PubMed
-
- World Health Organization (WHO) “Guidelines for drinking-water quality’’: Incorporating the first and second addenda. 3rd. Vol. 1. Geneva, Switzerland: World Health Organization (WHO); 2008.
LinkOut - more resources
Full Text Sources

