In Vivo, Proteomic, and In Silico Investigation of Sapodilla for Therapeutic Potential in Gastrointestinal Disorders
- PMID: 31886219
- PMCID: PMC6925776
- DOI: 10.1155/2019/4921086
In Vivo, Proteomic, and In Silico Investigation of Sapodilla for Therapeutic Potential in Gastrointestinal Disorders
Abstract
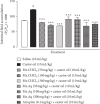
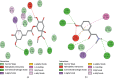
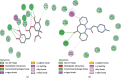
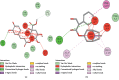
This study aims to delineate the effects of Manilkara zapota Linn. (Sapodilla) fruit chloroform (Mz.CHCl3) and aqueous (Mz.Aq) extracts tested through different techniques. Antidiarrheal activity and intestinal fluid accumulation were examined by using castor oil-induced diarrhea and castor oil fluid accumulation models. Isolated rabbit jejunum tissues were employed for in vitro experiments. Antimotility and antiulcer were performed through charcoal meal transient time and ethanol-induced ulcer assay, molecular studies were conducted through proteomic analysis, and virtual screening was performed by using a discovery studio visualizer (DSV). Mz.CHCl3 and Mz.Aq extracts attributed dose-dependent (50-300 mg/kg) protection (20-100%) against castor oil-induced diarrhea and dose-dependently (50-300 mg/kg) inhibited intestinal fluid secretions in mice. Mz.CHCl3 and Mz.Aq extracts produce relaxation of spontaneous and K+ (80 Mm) induced contractions in isolated tissue preparations and decreased the distance moved by charcoal in the gastrointestinal transit model in rats. It showed gastroprotective effect in ulcerative stomach of rats and decreased levels of IL-18 quantified by proteomic analysis. Histopathological results showed ethanol-induced significant gastric injury, leading to cloudy swelling, hydropic degeneration, apoptosis, and focal necrosis in all gastric zones using hematoxylin and eosin (H&E) staining. Moreover, ethanol increased the activation and the expression of tumor necrotic factor (TNF-α), cyclooxygenase (COX-2), and nuclear factor kappa-light-chain-enhancer of activated B cells (p-NFκB). In silico results were comparative to in vitro results evaluated through virtual screening. Moreover, ethanol increased the activation and expression of tumor necrotic factor, cyclooxygenase, and nuclear factor kappa-light-chain-enhancer of activated B cells. This study exhibits the gastroprotective effect of Manilkara zapota extracts in the peritoneal cavity using a proteomic and in silico approach which reveals different energy values against target proteins, which mediate the gastrointestinal functions.
Copyright © 2019 Sameen Fatima Ansari et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures








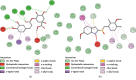
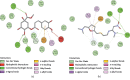
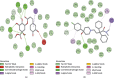
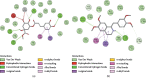
References
-
- Khan A.-u., Gilani A. H. Antidiarrheal, antisecretory, and bronchodilatory activities of Hypericum perforatum. Pharmaceutical Biology. 2009;47(10):962–967. doi: 10.1080/13880200902960206. - DOI
-
- Madani B., Mirshekari A., Yahia E., Golding J. B. Horticultural Reviews. 1. Vol. 45. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2018. Sapota (Manilkara achras Forb.): factors influencing fresh and processed fruit quality; pp. 105–142. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

