Data in support of the longitudinal characterization of pulmonary function in children with Mucopolysaccharidoses IVA
- PMID: 31886335
- PMCID: PMC6920461
- DOI: 10.1016/j.dib.2019.104756
Data in support of the longitudinal characterization of pulmonary function in children with Mucopolysaccharidoses IVA
Abstract
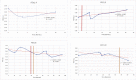
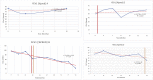
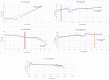
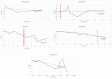
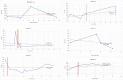
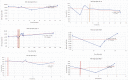
Mucopolysaccharidoses type IVA (Morquio disease) is a rare, autosomal recessive lysosomal storage disease that causes both obstructive and restrictive airway pathology, with respiratory failure being the primary cause of death. This article provides original data on the longitudinal characterization of pulmonary function changes in children with Mucopolysaccharidoses (MPS) IVA by presenting the data and nuanced trends of changes from sequential spirometry and oximetry. The sample size included 16 subjects, 13 had undergone enzyme replacement therapy (ERT), three had not undergone ERT treatment. A total of 180 individual plots are presented for spirometry variables (FEV1, FEV1 [%Pred] FVC, FVC [%Pred] and FEV1/FVC), 6MWT and oximetry variables (median %Spo2, ODI 3%, mean nadir 3%, ODI 4%, mean nadir 4% and min dip SpO2 [%]); over a nine-year period at a single quaternary paediatric metabolic centre. This data has been made public and has utility to clinicians and researchers due to the following: [1,2] by providing the first comprehensive report of detailed changes in pulmonary function in children with MPS IVA, with and without ERT; [1-3] as well as changes in pulmonary function following the institution of non-invasive ventilation (NIV) and adenotonsillectomy. The data presented is related to the research article by Kenth et al. "The Characterization of Pulmonary Function in Patients with Mucopolysaccharidoses IVA: A Longitudinal Analysis".
Keywords: Enzyme replacement therapy; MPS; Morquio syndrome; Mucopolysaccharidosis IVA; Respiratory changes; Sleep-disordered breathing.
Crown Copyright © 2019 Published by Elsevier Inc.
Figures











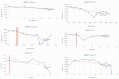

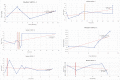


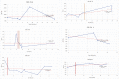


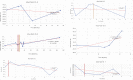









References
-
- Broomfield A., Kenth J., Bruce I.A., Hl T., Wilkinson S. Respiratory complications of metabolic disease in the paediatric population: a review of presentation, diagnosis and therapeutic options. Paediatr. Respir. Rev. 2019 - PubMed
-
- Jones S., Wynn R. Mucopolysaccharidoses: Clinical features and diagnosis - UpToDate. https://www.uptodate.com/contents/mucopolysaccharidoses-clinical-feature...
-
- Standardization of Spirometry Update. American thoracic society. Am. J. Respir. Crit. Care Med. 1994;152(1995):1107–1136. - PubMed
-
- Pal A.R., Langereis E.J., Saif M.A., Mercer J., Church H.J., Tylee K.L., Wynn R.F., Wijburg F.A., Jones S.A., Bruce I.A., Bigger B.W. Sleep disordered breathing in mucopolysaccharidosis I: a multivariate analysis of patient, therapeutic and metabolic correlators modifying long term clinical outcome. Orphanet J. Rare Dis. 2015;10:42. - PMC - PubMed
LinkOut - more resources
Full Text Sources

