Insights into the κ/ι-carrageenan metabolism pathway of some marine Pseudoalteromonas species
- PMID: 31886414
- PMCID: PMC6923384
- DOI: 10.1038/s42003-019-0721-y
Insights into the κ/ι-carrageenan metabolism pathway of some marine Pseudoalteromonas species
Abstract
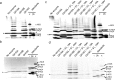
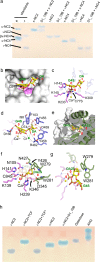
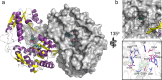
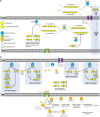
Pseudoalteromonas is a globally distributed marine-associated genus that can be found in a broad range of aquatic environments, including in association with macroalgal surfaces where they may take advantage of these rich sources of polysaccharides. The metabolic systems that confer the ability to metabolize this abundant form of photosynthetically fixed carbon, however, are not yet fully understood. Through genomics, transcriptomics, microbiology, and specific structure-function studies of pathway components we address the capacity of newly isolated marine pseudoalteromonads to metabolize the red algal galactan carrageenan. The results reveal that the κ/ι-carrageenan specific polysaccharide utilization locus (CarPUL) enables isolates possessing this locus the ability to grow on this substrate. Biochemical and structural analysis of the enzymatic components of the CarPUL promoted the development of a detailed model of the κ/ι-carrageenan metabolic pathway deployed by pseudoalteromonads, thus furthering our understanding of how these microbes have adapted to a unique environmental niche.
Keywords: Carbohydrates; Enzymes; Glycobiology; Structural biology; X-ray crystallography.
© The Author(s) 2019.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures



References
-
- Schaumann K, Weide G. Efficiency of uronic acid uptake in marine alginate-degrading fungi. Helgol.änder Meeresuntersuchungen. 1995;49:159–167. doi: 10.1007/BF02368346. - DOI
-
- Genilloud O, Peláez F, González I, Díez MT. Diversity of actinomycetes and fungi on seaweeds from the Iberian coasts. Microbiologia. 1994;10:413–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

