Very early Guillain-Barré syndrome: A clinical-electrophysiological and ultrasonographic study
- PMID: 31886449
- PMCID: PMC6923288
- DOI: 10.1016/j.cnp.2019.11.003
Very early Guillain-Barré syndrome: A clinical-electrophysiological and ultrasonographic study
Abstract
Objectives: Using recent optimized electrodiagnostic criteria sets, we primarily aimed at verifying the accuracy of the initial electrophysiological test in very early Guillain-Barré syndrome (VEGBS), ≤4 days of onset, compared with the results of serial electrophysiology. Our secondary objective was to correlate early electrophysiological results with sonographic nerve changes.
Methods: This is a retrospective study based on consecutive VEGBS patients admitted to the hospital. Each patient had serial nerve conduction studies (NCS) in at least 4 nerves. Initial NCS were done within 4 days after onset, and serial ones from the second week onwards. Electrophysiological recordings of each case were re-evaluated, GBS subtype being established accordingly. Nerve ultrasonography was almost always performed within 2 weeks after onset.
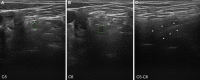
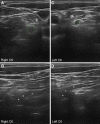
Results: Fifteen adult VEGBS patients were identified with a mean age of 57.8 years. At first NCS, VEGBS sub-typing was only possible in 3 (20%) cases that showed an axonal pattern, the remaining patterns being mixed (combining axonal and demyelinating features) in 6 (40%), equivocal in 5 (33.3%), and normal in 1 (6.7%). Upon serial NCS, 7 (46.7%) cases were categorized as acute demyelinating polyneuropathy, 7 (46.7%) as axonal GBS, and 1 (6.6%) as unclassified syndrome. Antiganglioside reactivity was detected in 5 out of the 7 axonal cases. Nerve US showed that lesions mainly involved the ventral rami of scanned cervical nerves.
Conclusions: Serial electrophysiological evaluation is necessary for accurate VEGBS subtype classification. Ultrasonography helps delineate the topography of nerve changes.
Significance: We provide new VEGBS pathophysiological insights into nerve conduction alterations within the first 4 days of the clinical course.
Keywords: Axonal degeneration; Demyelination; Endoneurial inflammatory oedema; Guillain-Barré syndrome; Ultrasonography; Very early Guillain-Barré syndrome.
© 2019 International Federation of Clinical Neurophysiology. Published by Elsevier B.V.
Figures


References
-
- Albertí M.A., Alentorn A., Martínez-Yelamos S., Martínez-Matos J.A., Povedano M., Montero J. Very early electrodiagnostic findings in Guillain-Barré syndrome. J. Peripher. Nerv. Syst. 2011;16:136–142. - PubMed
-
- Asbury A.K., Cornblath D.R. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann. Neurol. 1990;(27 Suppl):S21–S24. - PubMed
-
- Berciano J., García A., Figols J., Muñoz R., Berciano M.T., Lafarga M. Perineurium contributes to axonal damage in acute inflammatory demyelinating polyneuropathy. Neurology. 2000;55:552–559. - PubMed
-
- Berciano J., Gallardo E., Orizaola P., de Lucas E.M., García A., Pelayo-Negro A.L. Early axonal Guillain-Barré syndrome with normal peripheral conduction: imaging evidence for changes in proximal nerve segments. J. Neurol. Neurosurg. Psychiatry. 2016;87:563–565. - PubMed
-
- Berciano J., Sedano M.J., Pelayo-Negro A.L., García A., Orizaola P., Gallardo E. Proximal nerve lesions in early Guillain-Barré syndrome: implications for pathogenesis and disease classification. J. Neurol. 2017;264:221–236. - PubMed
LinkOut - more resources
Full Text Sources

