Myeloma Cells Down-Regulate Adiponectin in Bone Marrow Adipocytes Via TNF-Alpha
- PMID: 31886918
- PMCID: PMC9328417
- DOI: 10.1002/jbmr.3951
Myeloma Cells Down-Regulate Adiponectin in Bone Marrow Adipocytes Via TNF-Alpha
Abstract
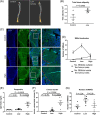
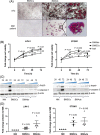
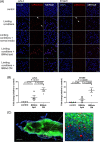
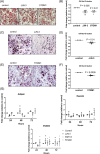
Multiple myeloma is caused by abnormal plasma cells that accumulate in the bone marrow and interact with resident cells of the bone microenvironment to drive disease progression and development of an osteolytic bone disease. Bone marrow adipocytes (BMAds) are emerging as having important endocrine functions that can support myeloma cell growth and survival. However, how BMAds respond to infiltrating tumor cells remains poorly understood. Using the C57BL/KaLwRij murine model of myeloma, bone marrow adiposity was found to be increased in early stage myeloma with BMAds localizing along the tumor-bone interface at later stages of disease. Myeloma cells were found to uptake BMAd-derived lipids in vitro and in vivo, although lipid uptake was not associated with the ability of BMAds to promote myeloma cell growth and survival. However, BMAd-derived factors were found to increase myeloma cell migration, viability, and the evasion of apoptosis. BMAds are a major source of adiponectin, which is known to be myeloma-suppressive. Myeloma cells were found to downregulate adiponectin specifically in a model of BMAds but not in white adipocytes. The ability of myeloma cells to downregulate adiponectin was dependent at least in part on TNF-α. Collectively our data support the link between increased bone marrow adiposity and myeloma progression. By demonstrating how TNF-α downregulates BMAd-derived adiponectin, we reveal a new mechanism by which myeloma cells alter the bone microenvironment to support disease progression. © 2019 The Authors. Journal of Bone and Mineral Research published by American Society for Bone and Mineral Research.
Keywords: ADIPOCYTE; ADIPONECTIN; BONE MARROW ADIPOSE TISSUE; CANCER; MULTIPLE MYELOMA.
© 2019 The Authors. Journal of Bone and Mineral Research published by American Society for Bone and Mineral Research.
Figures


Similar articles
-
Myeloma-Modified Adipocytes Exhibit Metabolic Dysfunction and a Senescence-Associated Secretory Phenotype.Cancer Res. 2021 Feb 1;81(3):634-647. doi: 10.1158/0008-5472.CAN-20-1088. Epub 2020 Nov 20. Cancer Res. 2021. PMID: 33218968 Free PMC article.
-
Myeloma and marrow adiposity: Unanswered questions and future directions.Best Pract Res Clin Endocrinol Metab. 2021 Jul;35(4):101541. doi: 10.1016/j.beem.2021.101541. Epub 2021 May 1. Best Pract Res Clin Endocrinol Metab. 2021. PMID: 34006450 Review.
-
Bone marrow adiposity and the hematopoietic niche: A historical perspective of reciprocity, heterogeneity, and lineage commitment.Best Pract Res Clin Endocrinol Metab. 2021 Jul;35(4):101564. doi: 10.1016/j.beem.2021.101564. Epub 2021 Aug 10. Best Pract Res Clin Endocrinol Metab. 2021. PMID: 34417114 Review.
-
Development and characterization of three cell culture systems to investigate the relationship between primary bone marrow adipocytes and myeloma cells.Front Oncol. 2023 Jan 11;12:912834. doi: 10.3389/fonc.2022.912834. eCollection 2022. Front Oncol. 2023. PMID: 36713534 Free PMC article.
-
Myeloma-bone marrow adipocyte axis in tumour survival and treatment response.Br J Cancer. 2021 Sep;125(6):775-777. doi: 10.1038/s41416-021-01371-4. Epub 2021 Apr 15. Br J Cancer. 2021. PMID: 33859343 Free PMC article.
Cited by
-
Correlation Between Bariatric Surgery and the Risk of Multiple Myeloma: Results from an Evidence-Based Strategy.Obes Surg. 2024 Apr;34(4):1061-1072. doi: 10.1007/s11695-024-07059-x. Epub 2024 Jan 17. Obes Surg. 2024. PMID: 38231452
-
Bone Marrow Adipocytes: A Link between Obesity and Bone Cancer.Cancers (Basel). 2021 Jan 20;13(3):364. doi: 10.3390/cancers13030364. Cancers (Basel). 2021. PMID: 33498240 Free PMC article. Review.
-
Deletion of Hsd11b1 suppresses caloric restriction-induced bone marrow adiposity in male but not female mice.J Endocrinol. 2024 Jun 24;262(2):e240072. doi: 10.1530/JOE-24-0072. Print 2024 Aug 1. J Endocrinol. 2024. PMID: 38805506 Free PMC article.
-
Myeloma-Modified Adipocytes Exhibit Metabolic Dysfunction and a Senescence-Associated Secretory Phenotype.Cancer Res. 2021 Feb 1;81(3):634-647. doi: 10.1158/0008-5472.CAN-20-1088. Epub 2020 Nov 20. Cancer Res. 2021. PMID: 33218968 Free PMC article.
-
Multiple Myeloma Cells Alter Adipogenesis, Increase Senescence-Related and Inflammatory Gene Transcript Expression, and Alter Metabolism in Preadipocytes.Front Oncol. 2021 Feb 18;10:584683. doi: 10.3389/fonc.2020.584683. eCollection 2020. Front Oncol. 2021. PMID: 33680918 Free PMC article.
References
-
- Mundy GR. Mechanisms of bone metastasis. Cancer. 1997;80(8 Suppl):1546–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

