Cross-talk between microglia and neurons regulates HIV latency
- PMID: 31887215
- PMCID: PMC6953890
- DOI: 10.1371/journal.ppat.1008249
Cross-talk between microglia and neurons regulates HIV latency
Abstract
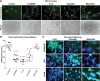
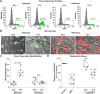
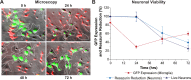
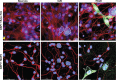
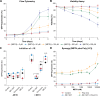
Despite effective antiretroviral therapy (ART), HIV-associated neurocognitive disorders (HAND) are found in nearly one-third of patients. Using a cellular co-culture system including neurons and human microglia infected with HIV (hμglia/HIV), we investigated the hypothesis that HIV-dependent neurological degeneration results from the periodic emergence of HIV from latency within microglial cells in response to neuronal damage or inflammatory signals. When a clonal hμglia/HIV population (HC69) expressing HIV, or HIV infected human primary and iPSC-derived microglial cells, were cultured for a short-term (24 h) with healthy neurons, HIV was silenced. The neuron-dependent induction of latency in HC69 cells was recapitulated using induced pluripotent stem cell (iPSC)-derived GABAergic cortical (iCort) and dopaminergic (iDopaNer), but not motor (iMotorNer), neurons. By contrast, damaged neurons induce HIV expression in latently infected microglial cells. After 48-72 h co-culture, low levels of HIV expression appear to damage neurons, which further enhances HIV expression. There was a marked reduction in intact dendrites staining for microtubule associated protein 2 (MAP2) in the neurons exposed to HIV-expressing microglial cells, indicating extensive dendritic pruning. To model neurotoxicity induced by methamphetamine (METH), we treated cells with nM levels of METH and suboptimal levels of poly (I:C), a TLR3 agonist that mimics the effects of the circulating bacterial rRNA found in HIV infected patients. This combination of agents potently induced HIV expression, with the METH effect mediated by the σ1 receptor (σ1R). In co-cultures of HC69 cells with iCort neurons, the combination of METH and poly(I:C) induced HIV expression and dendritic damage beyond levels seen using either agent alone, Thus, our results demonstrate that the cross-talk between healthy neurons and microglia modulates HIV expression, while HIV expression impairs this intrinsic molecular mechanism resulting in the excessive and uncontrolled stimulation of microglia-mediated neurotoxicity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

