A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety and speed of response from a randomized, double-blinded trial
- PMID: 31887225
- PMCID: PMC7317420
- DOI: 10.1111/bjd.18851
A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety and speed of response from a randomized, double-blinded trial
Abstract
Background: Patients with psoriasis value rapid and complete skin clearance. No head-to-head studies have focused on early responses to interleukin (IL)-17 vs. IL-23 inhibitors.
Objectives: To compare early and complete skin clearance by the IL-17A inhibitor ixekizumab vs. the IL-23p19 inhibitor guselkumab.
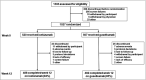
Methods: IXORA-R, a 24-week, randomized, double-blinded study, enrolled adults with moderate-to-severe plaque psoriasis [static Physician's Global Assessment of Disease (sPGA) score of ≥ 3, Psoriasis Area and Severity Index (PASI) ≥ 12, and ≥ 10% body surface area]. Patients were randomized (1 : 1) to receive the approved dose of subcutaneous ixekizumab or guselkumab. Primary end point was 100% improvement in PASI (PASI 100) at week 12. Major secondary end points included other levels of improved PASI and sPGA at different time points. Comparisons were made using the Cochran-Mantel-Haenszel test with a multiple testing strategy. Nonresponder imputation was used for missing data. After the completion of the study, the final secondary end point (PASI 100 at 24 weeks) and safety data through week 24 will be reported.
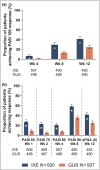
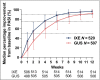
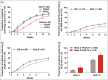
Results: In total, 1027 patients were randomized. The primary end point PASI 100 at week 12 was met [215/520 ixekizumab (41%); 126/507 guselkumab (25%); P < 0·001]. All major secondary end points measured up to week 12 were met, including PASI 50 at week 1 and PASI 75 at week 2. Serious adverse event frequency was 3% for each group; no new safety signals were identified.
Conclusions: Ixekizumab was superior to guselkumab for rapidly improving signs and symptoms in patients with moderate-to-severe plaque psoriasis by week 12. Adverse events were similar to previous ixekizumab and guselkumab studies. Compared with the IL-23 inhibitor guselkumab, ixekizumab can offer complete skin clearance more rapidly to patients with moderate-to-severe plaque psoriasis. What's already known about this topic? Patients with plaque psoriasis desire both high levels of clearance and rapid onset of treatment effects. Ixekizumab, a high-affinity monoclonal antibody that selectively targets interleukin (IL)-17A, has demonstrated greater and faster skin clearance than etanercept and ustekinumab, with consistent long-term efficacy, safety and durability of response. Clinical trial data and systematic reviews have suggested that IL-17 inhibitors can improve a patient's psoriasis more rapidly than IL-23 inhibitors. What does this study add? The head-to-head study design directly compares the efficacy and speed of response of ixekizumab and the IL-23 inhibitor guselkumab in moderate-to-severe plaque psoriasis. The primary end point was met, showing superiority of ixekizumab over guselkumab for achieving complete skin clearance at week 12. The safety profile of ixekizumab was consistent with previous studies. Ixekizumab can deliver patients complete skin clearance and improved quality of life more rapidly than guselkumab.
© 2019 The Authors. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Figures
Comment in
-
Choosing a biologic for psoriasis: is it a sprint or a marathon?Br J Dermatol. 2020 Jun;182(6):1321-1322. doi: 10.1111/bjd.19038. Epub 2020 Apr 13. Br J Dermatol. 2020. PMID: 32285438 No abstract available.
References
-
- Boehncke WH, Schon MP. Psoriasis. Lancet 2015; 386:983–94. - PubMed
-
- Warren RB, Kleyn CE, Gulliver WP. Cumulative life course impairment in psoriasis: patient perception of disease‐related impairment throughout the life course. Br J Dermatol 2011; 164 (Suppl 1):1–14. - PubMed
-
- Blome C, Gosau R, Radtke MA et al Patient‐relevant treatment goals in psoriasis. Arch Dermatolog Res 2016; 308:69–78. - PubMed
-
- Carrascosa JM, de la Cueva P, Herranz P et al Perception of psoriasis treatment in the outpatient setting: survey of patients and their prescribing physicians. J Dermatol Treat 2017; 28:188–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

