Outcomes based on age in the phase III METEOR trial of cabozantinib versus everolimus in patients with advanced renal cell carcinoma
- PMID: 31887537
- PMCID: PMC7521477
- DOI: 10.1016/j.ejca.2019.10.032
Outcomes based on age in the phase III METEOR trial of cabozantinib versus everolimus in patients with advanced renal cell carcinoma
Abstract
Background: Cabozantinib improved progression-free survival (PFS), overall survival (OS) and objective response rate (ORR) compared with everolimus in patients with advanced renal cell carcinoma (RCC) after prior antiangiogenic therapy in the phase III METEOR trial (NCT01865747). Limited data are available on the use of targeted therapies in older patients with advanced RCC.
Methods: Efficacy and safety in METEOR were retrospectively analysed for three age subgroups: <65 (n = 394), 65-74 (n = 201) and ≥75 years (n = 63).
Results: PFS, OS and ORR were improved with cabozantinib compared with everolimus in all age subgroups. The PFS hazard ratios (HRs) were 0.53 (95% confidence interval [CI]: 0.41-0.68), 0.53 (95% CI: 0.37-0.77) and 0.38 (95% CI: 0.18-0.79) for <65, 65-74 and ≥75 years, respectively, and the OS HRs were 0.72 (95% CI: 0.54-0.95), 0.66 (95% CI: 0.44-0.99) and 0.57 (95% CI: 0.28-1.14). The ORR for cabozantinib versus everolimus was 15% vs 5%, 21% vs 2% and 19% vs 0%, respectively. No significant differences were observed in PFS or OS with age as a categorical or continuous variable. Grade III/IV adverse events (AEs) were generally consistent across subgroups, although fatigue, hypertension and hyponatraemia occurred more frequently in older patients treated with cabozantinib. Dose reductions to manage AEs were more frequent in patients receiving cabozantinib than in those receiving everolimus. Dose reductions and treatment discontinuation due to AEs were more frequent in older patients in both treatment groups.
Conclusions: Cabozantinib improved PFS, OS and ORR compared with everolimus in previously treated patients with advanced RCC, irrespective of age group, supporting use in all age categories. Proactive dose modification and supportive care may help to mitigate AEs in older patients while maintaining efficacy.
Keywords: Age; Cabozantinib; Everolimus; METEOR; Renal cell carcinoma; Tyrosine kinase inhibitor; Vascular endothelial growth factor receptor.
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflict of interest statement F.D. certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g. employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony royalties or patents filed, received or pending), are the following: F.D. received research grant support from Pfizer, Novartis, Ipsen, and Health Research Foundation of Central Denmark Region, outside the submitted work. R.J.M. served in a consultancy or advisory role for Pfizer, Novartis, Merck, Genentech/Roche, Eisai and Exelixis and received research funding from Bristol-Myers Squibb, Pfizer, Genentech/Roche, Eisai, Exelixis and Novartis. K.C. received honoraria from Roche, served in a consultancy or advisory role for Roche and Novartis and received support for travel and accommodation from Roche and Amgen. S.N. received honoraria from Pfizer, Bristol-Myers Squibb, Novartis, Ipsen and Eusa Pharma, outside the submitted work. I.C. and A.C. are employees of Exelixis. B.E. received grant support and personal fees from Bristol-Myers Squibb, Pfizer, Novartis, Ipsen and Eusa. S.P. received honoraria from Astellas Pharma, Medivation and Novartis, served in a consultancy or advisory role for Aveo, Bristol-Myers Squibb, Exelixis, Genentech, Myriad Pharmaceuticals, Novartis and Pfizer and received research funding from Medivation. T.P. served in a consultancy or advisory role for AstraZeneca, Bristol-Myers Squibb, Genentech/Roche, Merck and Novartis, and received research funding from AstraZeneca/MedImmune and Roche/Genentech. T.K.C. served in a consultancy or advisory role for Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Laboratories, Corvus, Ipsen, UpToDate, NCCN and Analysis Group, received research funding (institutional and personal) from AstraZeneca, Bayer, BMS, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Ipsen, Tracon, Genentech, Roche, Roche Products Limited, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, Prometheus Laboratories, Corvus, Calithera, Analysis Group and Takeda, received honoraria from AstraZeneca, Alexion, Sanofi/Aventis, Bayer, BMS, Cerulean, Eisai, Foundation Medicine Inc., Exelixis, Genentech, Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Laboratories, Corvus, Ipsen, UpToDate, NCCN, Analysis Group, NCCN, Michael J. Hennessy (MJH) Associates, Inc (Healthcare Communications Company with several brands such as OncLive and PER), Lpath, Kidney Cancer journal, Clinical Care Options, PlatformQ, Navinata Healthcare, Harborside Press, American Society of Medical Oncology, NEJM, Lancet Oncology and Heron Therapeutics and has received travel, accommodations and expenses in relation to consulting and advisory roles or honoraria; T.K.C.'s institution (Dana-Farber Cancer Institute) may have received additional independent funding or royalties from drug companies potentially involved in research around the subject matter. All other authors declare no conflict of interest.
Figures
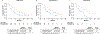
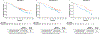
References
-
- Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 2015;67(3):519–30. - PubMed
-
- Kanesvaran R, Saux OL, Motzer R, Choueiri TK, Scotte F, Bellmunt J, et al. Elderly patients with metastatic renal cell carcinoma: position paper from the International Society of Geriatric Oncology. Lancet Oncol 2018;19(6):e317–26. - PubMed
-
- Verhoest G, Veillard D, Guille F, De La Taille A, Salomon L, Abbou CC, et al. Relationship between age at diagnosis and clinicopathologic features of renal cell carcinoma. Eur Urol 2007; 51(5):1298–304. discussion 1304–5. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

