Collaboration Between RSK-EphA2 and Gas6-Axl RTK Signaling in Arginine Starvation Response That Confers Resistance to EGFR Inhibitors
- PMID: 31887630
- PMCID: PMC6938815
- DOI: 10.1016/j.tranon.2019.12.003
Collaboration Between RSK-EphA2 and Gas6-Axl RTK Signaling in Arginine Starvation Response That Confers Resistance to EGFR Inhibitors
Abstract
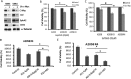
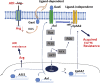
Many human malignancies require extracellular arginine (Arg) for survival because the key enzyme for de novo Arg biosynthesis, argininosuccinate synthetase 1 (ASS1), is silenced. Recombinant arginine deiminase (ADI-PEG20), which digests extracellular Arg, has been in clinical trials for treating ASS1-negative tumors. Reactivation of ASS1 is responsible for the treatment failure. We previously demonstrated that ASS1 reactivation is transcriptionally regulated by c-Myc via the upstream Gas6-Axl tyrosine kinase (RTK) signal. Here, we report that another RTK EphA2 is coactivated via PI3K-ERK/RSK1 pathway in a ligand-independent mechanism. EphA2 is also regulated by c-Myc. Moreover, we found that knockdown Axl upregulates EphA2 expression, demonstrating cross-talk between these RTKs. ADIR cell lines exhibits enhanced sensitivities to nutrient deprivation such as charcoal-stripped FBS and multiple RTK inhibitor foretinib but resistance to EGFR inhibitors. Knockdown EphA2, and to lesser extent, Axl, overcomes EGFRi resistance. c-Myc inhibitor JQ1 can also sensitize ADIR cells to ADI-PEG20. This study elucidates molecular interactions of multiple RTKs in Arg-stress response and offers approaches for developing strategies of overcoming ADI-PEG20 resistance.
Published by Elsevier Inc.
Figures





References
-
- Closs E.I., Simon A., Vekony N., Rotmann A. Plasma membrane transporters for arginine. J Nutr. 2004;134:2752S–2759S. discussion 2765S-2767S. - PubMed
-
- Dillon B.J., Prieto V.G., Curley S.A., Ensor C.M., Holtsberg F.W., Bomalaski J.S., Clark M.A. Incidence and distribution of argininosuccinate synthetase deficiency in human cancers: a method for identifying cancers sensitive to arginine deprivation. Cancer. 2004;100:826–833. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

