Human Plasma-like Medium Improves T Lymphocyte Activation
- PMID: 31887663
- PMCID: PMC6941860
- DOI: 10.1016/j.isci.2019.100759
Human Plasma-like Medium Improves T Lymphocyte Activation
Abstract
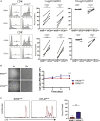
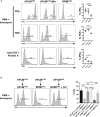
T lymphocytes are critical for effective immunity, and the ability to study their behavior in vitro can facilitate major insights into their development, function, and fate. However, the composition of human plasma differs from conventional media, and we hypothesized that such differences could impact immune cell physiology. Here, we showed that relative to the medium typically used to culture lymphocytes (RPMI), a physiologic medium (human plasma-like medium; HPLM) induced markedly different transcriptional responses in human primary T cells and in addition, improved their activation upon antigen stimulation. We found that this medium-dependent effect on T cell activation is linked to Ca2+, which is six-fold higher in HPLM than in RPMI. Thus, a medium that more closely resembles human plasma has striking effects on T cell biology, further demonstrates that medium composition can profoundly affect experimental results, and broadly suggests that physiologic media may offer a valuable way to study cultured immune cells.
Keywords: Biological Sciences; Immunological Methods; Immunology.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures




References
-
- Berod L., Friedrich C., Nandan A., Freitag J., Hagemann S., Harmrolfs K., Sandouk A., Hesse C., Castro C.N., Bähre H. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 2014;20:1327–1333. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

