Accurate and sensitive quantitation of glucose and glucose phosphates derived from storage carbohydrates by mass spectrometry
- PMID: 31887930
- PMCID: PMC7018519
- DOI: 10.1016/j.carbpol.2019.115651
Accurate and sensitive quantitation of glucose and glucose phosphates derived from storage carbohydrates by mass spectrometry
Abstract
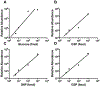
The addition of phosphate groups into glycogen modulates its branching pattern and solubility which all impact its accessibility to glycogen interacting enzymes. As glycogen architecture modulates its metabolism, it is essential to accurately evaluate and quantify its phosphate content. Simultaneous direct quantitation of glucose and its phosphate esters requires an assay with high sensitivity and a robust dynamic range. Herein, we describe a highly-sensitive method for the accurate detection of both glycogen-derived glucose and glucose-phosphate esters utilizing gas-chromatography coupled mass spectrometry. Using this method, we observed higher glycogen levels in the liver compared to skeletal muscle, but skeletal muscle contained many more phosphate esters. Importantly, this method can detect femtomole levels of glucose and glucose phosphate esters within an extremely robust dynamic range with excellent accuracy and reproducibility. The method can also be easily adapted for the quantification of plant starch, amylopectin or other biopolymers.
Keywords: GCMS; Glucose; Glucose phosphate esters; Glycogen; Lafora disease; Laforin; Starch.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Muscle glycogen remodeling and glycogen phosphate metabolism following exhaustive exercise of wild type and laforin knockout mice.J Biol Chem. 2015 Sep 11;290(37):22686-98. doi: 10.1074/jbc.M115.673897. Epub 2015 Jul 27. J Biol Chem. 2015. PMID: 26216881 Free PMC article.
-
Glycogen phosphomonoester distribution in mouse models of the progressive myoclonic epilepsy, Lafora disease.J Biol Chem. 2015 Jan 9;290(2):841-50. doi: 10.1074/jbc.M114.607796. Epub 2014 Nov 21. J Biol Chem. 2015. PMID: 25416783 Free PMC article.
-
Laforin is a glycogen phosphatase, deficiency of which leads to elevated phosphorylation of glycogen in vivo.Proc Natl Acad Sci U S A. 2007 Dec 4;104(49):19262-6. doi: 10.1073/pnas.0707952104. Epub 2007 Nov 26. Proc Natl Acad Sci U S A. 2007. PMID: 18040046 Free PMC article.
-
Glycogen phosphorylation and Lafora disease.Mol Aspects Med. 2015 Dec;46:78-84. doi: 10.1016/j.mam.2015.08.003. Epub 2015 Aug 13. Mol Aspects Med. 2015. PMID: 26278984 Free PMC article. Review.
-
Lafora disease offers a unique window into neuronal glycogen metabolism.J Biol Chem. 2018 May 11;293(19):7117-7125. doi: 10.1074/jbc.R117.803064. Epub 2018 Feb 26. J Biol Chem. 2018. PMID: 29483193 Free PMC article. Review.
Cited by
-
Polyglucosan body structure in Lafora disease.Carbohydr Polym. 2020 Jul 15;240:116260. doi: 10.1016/j.carbpol.2020.116260. Epub 2020 Apr 14. Carbohydr Polym. 2020. PMID: 32475552 Free PMC article.
-
The Role of Acyl-CoA Synthetase 1 in Bioactive Lipid Accumulation and the Development of Hepatic Insulin Resistance.Nutrients. 2024 Mar 29;16(7):1003. doi: 10.3390/nu16071003. Nutrients. 2024. PMID: 38613036 Free PMC article.
-
A non-catalytic scaffolding activity of hexokinase 2 contributes to EMT and metastasis.Nat Commun. 2022 Feb 16;13(1):899. doi: 10.1038/s41467-022-28440-3. Nat Commun. 2022. PMID: 35173161 Free PMC article.
-
Metabolic and transcriptomic reprogramming during contact inhibition-induced quiescence is mediated by YAP-dependent and YAP-independent mechanisms.Nat Commun. 2024 Aug 8;15(1):6777. doi: 10.1038/s41467-024-51117-y. Nat Commun. 2024. PMID: 39117624 Free PMC article.
-
Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging of Glycogen In Situ.Methods Mol Biol. 2022;2437:215-228. doi: 10.1007/978-1-0716-2030-4_15. Methods Mol Biol. 2022. PMID: 34902151
References
-
- Agius L (2015). Role of glycogen phosphorylase in liver glycogen metabolism. Molecular Aspects of Medicine, 46, 34–45. - PubMed
-
- Baunsgaard L, Lütken H, Mikkelsen R, Glaring MA, Pham TT, & Blennow A (2005). A novel isoform of glucan, water dikinase phosphorylates pre- phosphorylated α- glucans and is involved in starch degradation in Arabidopsis. The Plant Journal, 41(4), 595–605. - PubMed
-
- Blennow A (2015). Phosphorylation of the starch granule In Starch (pp. 399–424): Springer
-
- Blennow A, & Engelsen SB (2010). Helix-breaking news: fighting crystalline starch energy deposits in the cell. Trends Plant Sci, 15(4), 236–240. - PubMed
-
- Brown AM, & Ransom BR (2007). Astrocyte glycogen and brain energy metabolism. Glia, 55(12), 1263–1271. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

