Consumption of Terpenoids-Rich Padina pavonia Extract Attenuates Hyperglycemia, Insulin Resistance and Oxidative Stress, and Upregulates PPARγ in a Rat Model of Type 2 Diabetes
- PMID: 31887984
- PMCID: PMC7022299
- DOI: 10.3390/antiox9010022
Consumption of Terpenoids-Rich Padina pavonia Extract Attenuates Hyperglycemia, Insulin Resistance and Oxidative Stress, and Upregulates PPARγ in a Rat Model of Type 2 Diabetes
Abstract
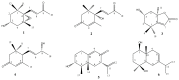
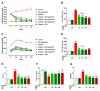
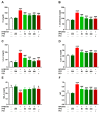
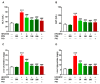
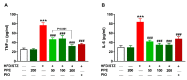
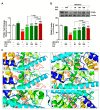
Seaweeds are rich in structurally diverse bioactive compounds with promising therapeutic effects. This study aimed to isolate and identify terpenes from the brown alga Padina pavonia and to investigate its antidiabetic activity, pointing to the possible involvement of peroxisome proliferator-activated receptor (PPAR)γ. Type 2 diabetes was induced by feeding rats a high fat diet (HFD) for 4 weeks followed by injection of 35 mg/kg streptozotocin (STZ). The diabetic rats received P. pavonia extract (PPE; 50, 100 and 200 mg/kg) for 4 weeks and samples were collected for analyses. HFD/STZ-induced rats showed hyperglycemia, dyslipidemia, impaired glucose tolerance, decreased insulin, and increased HbA1c and HOMA-IR. PPE ameliorated hyperglycemia and dyslipidemia, and improved glucose tolerance and insulin sensitivity in diabetic rats. Treatment with PPE increased hepatic hexokinase activity and glycogen, suppressed glucose-6-phosphatase, fructose-1,6-biphosphatase, and glycogen phosphorylase, and attenuated oxidative stress, inflammation, and liver injury and lipid infiltration in HFD/STZ-induced rats. In addition, PPE boosted antioxidants and upregulated PPARγ gene and protein expression in the liver of diabetic rats. Phytochemical investigation resulted in the isolation of six terpenes from PPE and in silico analysis revealed their binding affinity toward PPARγ. In conclusion, P. pavonia-derived terpenes attenuated hyperglycemia, dyslipidemia, oxidative stress, and inflammation, and improved insulin sensitivity and carbohydrate metabolism in type 2 diabetic rats. These beneficial effects are mediated via PPARγ activation. However, further studies to explore the exact mechanisms underlying the antidiabetic effect of PPE are recommended.
Keywords: PPARs; diabetes; oxidative stress; seaweeds; terpenes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

