Hybrid Material Based on an Amorphous-Carbon Matrix and ZnO/Zn for the Solar Photocatalytic Degradation of Basic Blue 41
- PMID: 31888030
- PMCID: PMC6983089
- DOI: 10.3390/molecules25010096
Hybrid Material Based on an Amorphous-Carbon Matrix and ZnO/Zn for the Solar Photocatalytic Degradation of Basic Blue 41
Abstract
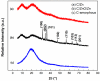
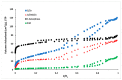
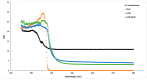
Innovative composites based on an amorphous-carbon matrix containing a second phase ZnO oxide and/or highly dispersed Zn metallic were synthesized via a modified Pechini route, in which a partial pyrolysis method was reached. Studies of adsorption in the dark and the photocatalytic activity for the cationic azo-dye, basic blue 41, and degradation were carried out. X-ray diffraction patterns for the carbon matrix and its composite with Zn show characteristics of the amorphous carbon. The infrared in the mid region of the composite prepared with ZnO and Zn exhibit vibrational bands related to bonds zinc oxide. The surface pH of the material is the main factor responsible for the adsorption of the azo-dye, but the contribution of mesopores favored the diffusion of molecules from the bulk of solution to the pore framework. Esters-like functional groups on the surface of carbons hinder the adsorption of the azo-dye. When Zn is embedded within amorphous carbon the photocatalytic activity of the composites showed up to 2.4 higher than neat ZnO. The enhancement in the photocatalytic activity and stability of C/ZnO/Zn and C/Zn composites is discussed in terms of a protector effect by the carbon layers inserted in composites. Carbon layers are responsible to inhibit the lixiviation of ZnO particles along irradiation.
Keywords: amorphous carbon; basic blue 41; carbon/ZnO composites; lixiviation; photocatalytic degradation; solar irradiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Investigation on solar photocatalytic degradation of various dyes in the presence of Er(3+):YAlO(3)/ZnO-TiO(2) composite.J Environ Manage. 2010 Jan-Feb;91(3):677-84. doi: 10.1016/j.jenvman.2009.09.031. Epub 2009 Oct 28. J Environ Manage. 2010. PMID: 19846250
-
Enhanced photocatalytic degradation of lindane using metal-semiconductor Zn@ZnO and ZnO/Ag nanostructures.J Environ Sci (China). 2018 Dec;74:107-115. doi: 10.1016/j.jes.2018.02.014. Epub 2018 Mar 3. J Environ Sci (China). 2018. PMID: 30340663
-
Enhanced photocatalytic degradation of methyl orange by porous graphene/ZnO nanocomposite.Environ Pollut. 2019 Jun;249:801-811. doi: 10.1016/j.envpol.2019.03.071. Epub 2019 Mar 28. Environ Pollut. 2019. PMID: 30953942
-
Synthesis and enhancement of photocatalytic activities of ZnO by silver nanoparticles.Spectrochim Acta A Mol Biomol Spectrosc. 2014 Mar 25;122:113-7. doi: 10.1016/j.saa.2013.09.116. Epub 2013 Oct 19. Spectrochim Acta A Mol Biomol Spectrosc. 2014. PMID: 24299983
-
A comprehensive review on crosslinked network systems of zinc oxide-organic polymer composites.Int J Biol Macromol. 2024 Aug;274(Pt 1):133250. doi: 10.1016/j.ijbiomac.2024.133250. Epub 2024 Jun 20. Int J Biol Macromol. 2024. PMID: 38908628 Review.
Cited by
-
Microwave-assisted synthesis of titania-amorphous carbon nanotubes/amorphous nitrogen-doped carbon nanotubes nanohybrids for photocatalytic degradation of textile wastewater.RSC Adv. 2021 Feb 8;11(12):6748-6763. doi: 10.1039/d0ra08191d. eCollection 2021 Feb 4. RSC Adv. 2021. PMID: 35423199 Free PMC article.
-
Removal of Malachite Green Dye from Aqueous Solution by Catalytic Wet Oxidation Technique Using Ni/Kaolin as Catalyst.Molecules. 2022 Nov 3;27(21):7528. doi: 10.3390/molecules27217528. Molecules. 2022. PMID: 36364350 Free PMC article.
References
-
- Hou X., Liu J., Zhang D., Zhao M., Xia C. Impact of urbanization on the eco-efficiency of cultivated land utilization: A case study on the Yangtze River Economic Belt, China. J. Clean. Prod. 2019;238:117916. doi: 10.1016/j.jclepro.2019.117916. - DOI
-
- Alshabib M., Onaizi S.A. A review on phenolic wastewater remediation using homogeneous and heterogeneous enzymatic processes: Current status and potential challenges. Sep. Purif. Technol. 2019;219:186–207. doi: 10.1016/j.seppur.2019.03.028. - DOI
MeSH terms
Substances
Grants and funding
- PIA/APOYO CCTE AFB170007/Comisión Nacional de Investigación Científica y Tecnológica
- Project 1190591/Fondo Nacional de Desarrollo Científico y Tecnológico
- Millennium Science Initiative of the Ministry of Economy, Development and Tourism, Chile, grant Nuclei on Catalytic Processes towards Sustainable Chemistry (CSC)/Instituto Millenium
- Grant# 02/05997-9/Fundação de Amparo à Pesquisa do Estado de São Paulo
- Grant # 07/03510-9/Fundação de Amparo à Pesquisa do Estado de São Paulo
LinkOut - more resources
Full Text Sources

