Immunomodulation of Murine Chronic DSS-Induced Colitis by Tuftsin-Phosphorylcholine
- PMID: 31888063
- PMCID: PMC7019495
- DOI: 10.3390/jcm9010065
Immunomodulation of Murine Chronic DSS-Induced Colitis by Tuftsin-Phosphorylcholine
Abstract
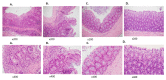
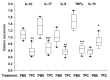
Helminths or their products can immunomodulate the host immune system, and this phenomenon may be applied as the basis of new anti-inflammatory treatments. Previously, we have shown the efficacy of tuftsin-phosphorylcholine (TPC), based on a helminth product, in four animal models of autoimmune diseases: arthritis, colitis, systemic lupus erythematosus, and experimental autoimmune encephalomyelitis. We demonstrated that TPC reduced inflammatory process ex vivo in peripheral blood lymphocytes (PBLs) and in biopsies from giant-cell arteritis. In the present study, we assessed the therapeutic potential of TPC treatment on a chronic colitis murine model. C57BL/6 mice with chronic colitis were treated with TPC after the third cycle of 2% dextran sodium sulfate (DSS). Oral TPC treatment resulted in amelioration of the colitis clinical manifestations exemplified by reduced disease activity index (DAI) score, expansion of mesenteric lymph nodes (MLN) T regulatory cells (shown by Fluorescence Activated Cell Sorting (FACS)), significant reduction in the expression of pro-inflammatory cytokines (IL-1β, IL17, IL-6, TNFα), and elevation in the expression of anti-inflammatory cytokine IL-10 (shown by RT-PCR). This study demonstrated the potential immunomodulatory effects of oral administration of TPC in a chronic colitis murine model. Further clinical trials are needed in order to evaluate this novel approach for the treatment of patients with inflammatory bowel disease.
Keywords: colitis; inflammatory bowel disease; phosphorylcholine; tuftsin.
Conflict of interest statement
Yehuda Shoenfeld and Miri Blank have shares in TPCera LTD.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

