TDP-43-Mediated Toxicity in HEK293T Cells: A Fast and Reproducible Protocol To Be Employed in the Search of New Therapeutic Options against Amyotrophic Lateral Sclerosis
- PMID: 31888078
- PMCID: PMC7016571
- DOI: 10.3390/cells9010068
TDP-43-Mediated Toxicity in HEK293T Cells: A Fast and Reproducible Protocol To Be Employed in the Search of New Therapeutic Options against Amyotrophic Lateral Sclerosis
Abstract
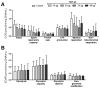
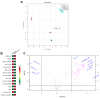
Cytoplasmic TDP-43 aggregates are a hallmark of amyotrophic lateral sclerosis (ALS). Today, only two drugs are available for ALS treatment, and their modest effect prompts researchers to search for new therapeutic options. TDP-43 represents one of the most promising targets for therapeutic intervention, but reliable and reproducible in vitro protocols for TDP-43-mediated toxicity are lacking. Here, we used HEK293T cells transfected with increasing concentrations of TDP-43-expressing plasmid to evaluate different parameters of toxicity and alterations in cellular metabolism. Overexpression of TDP-43 induced aggregates occurrence followed by the detection of 25- and 35-kDa forms of TDP-43. TDP-43 overexpression decreased cell viability and increased cells arrested at G2/M phase and nuclear fragmentation. Analysis of the energetic metabolism showed a tendency to decrease oxidative phosphorylation and increase glycolysis, but no statistical differences were observed. Metabolomics revealed alterations in different metabolites (mainly sphingolipids and glycerophospholipids) in cells overexpressing TDP-43. Our data reveal the main role of TDP-43 aggregation in cellular death and highlight novel insight into the mechanism of cellular toxicity induced by TDP-43. Here, we provide a simple, sensitive, and reliable protocol in a human-derived cell line to be used in high-throughput screenings of potential therapeutic molecules for ALS treatment.
Keywords: ALS; TDP-43; aggregation; apoptosis; cellular death; metabolomics.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Conditioned Medium from Cells Overexpressing TDP-43 Alters the Metabolome of Recipient Cells.Cells. 2020 Sep 29;9(10):2198. doi: 10.3390/cells9102198. Cells. 2020. PMID: 33003404 Free PMC article.
-
The debated toxic role of aggregated TDP-43 in amyotrophic lateral sclerosis: a resolution in sight?Brain. 2019 May 1;142(5):1176-1194. doi: 10.1093/brain/awz078. Brain. 2019. PMID: 30938443 Free PMC article. Review.
-
Tdp-25 Routing to Autophagy and Proteasome Ameliorates its Aggregation in Amyotrophic Lateral Sclerosis Target Cells.Sci Rep. 2018 Aug 17;8(1):12390. doi: 10.1038/s41598-018-29658-2. Sci Rep. 2018. PMID: 30120266 Free PMC article.
-
Thioridazine reverts the phenotype in cellular and Drosophila models of amyotrophic lateral sclerosis by enhancing TDP-43 aggregate clearance.Neurobiol Dis. 2021 Dec;160:105515. doi: 10.1016/j.nbd.2021.105515. Epub 2021 Sep 24. Neurobiol Dis. 2021. PMID: 34571136
-
TDP-43: A Key Therapeutic Target beyond Amyotrophic Lateral Sclerosis.ACS Chem Neurosci. 2019 Mar 20;10(3):1183-1196. doi: 10.1021/acschemneuro.9b00026. Epub 2019 Mar 4. ACS Chem Neurosci. 2019. PMID: 30785719 Review.
Cited by
-
Lipid alterations in human frontal cortex in ALS-FTLD-TDP43 proteinopathy spectrum are partly related to peroxisome impairment.Neuropathol Appl Neurobiol. 2021 Jun;47(4):544-563. doi: 10.1111/nan.12681. Epub 2021 Jan 12. Neuropathol Appl Neurobiol. 2021. PMID: 33332650 Free PMC article.
-
Conditioned Medium from Cells Overexpressing TDP-43 Alters the Metabolome of Recipient Cells.Cells. 2020 Sep 29;9(10):2198. doi: 10.3390/cells9102198. Cells. 2020. PMID: 33003404 Free PMC article.
-
Understanding In Vitro Pathways to Drug Discovery for TDP-43 Proteinopathies.Int J Mol Sci. 2022 Nov 25;23(23):14769. doi: 10.3390/ijms232314769. Int J Mol Sci. 2022. PMID: 36499097 Free PMC article.
-
Glycerophospholipids in ALS: insights into disease mechanisms and clinical implication.Mol Neurodegener. 2025 Jul 26;20(1):85. doi: 10.1186/s13024-025-00876-3. Mol Neurodegener. 2025. PMID: 40713843 Free PMC article. Review.
-
scFv intrabody targeting wildtype TDP-43 presents protective effects in a cellular model of TDP-43 proteinopathy.PLoS One. 2025 Aug 4;20(8):e0322021. doi: 10.1371/journal.pone.0322021. eCollection 2025. PLoS One. 2025. PMID: 40758691 Free PMC article.
References
-
- Polymenidou M., Lagier-Tourenne C., Hutt K.R., Huelga S.C., Moran J., Liang T.Y., Ling S.C., Sun E., Wancewicz E., Mazur C., et al. Long pre-MRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat. Neurosci. 2011;14:459–468. doi: 10.1038/nn.2779. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

