Ipragliflozin Ameliorates Endoplasmic Reticulum Stress and Apoptosis through Preventing Ectopic Lipid Deposition in Renal Tubules
- PMID: 31888083
- PMCID: PMC6981520
- DOI: 10.3390/ijms21010190
Ipragliflozin Ameliorates Endoplasmic Reticulum Stress and Apoptosis through Preventing Ectopic Lipid Deposition in Renal Tubules
Abstract
Background: Chronic kidney disease (CKD) and non-alcoholic steatohepatitis (NASH) are major health burdens closely related to metabolic syndrome. A link between CKD and NASH has been assumed; however, the underlying mechanism is still unknown. Ectopic lipid deposition (ELD) in the hepatocyte results in endoplasmic reticulum (ER) stress, which plays an important role in the development of steatohepatitis. ELD is also assumed to play a role in the development of kidney injury. We aimed to investigate the role of ELD and ER stress in the development of CKD, and evaluate the efficacy of a sodium glucose cotransporter-2 inhibitor, ipragliflozin.
Methods: Male FLS-ob/ob mice that closely imitate the pathophysiology of NASH were treated with vehicle or ipragliflozin. Metabolic characteristics, histology of the kidney, ER stress, and apoptotic signals were evaluated.
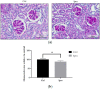
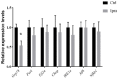
Results: The serum triglyceride was significantly lower in mice treated with ipragliflozin. Ipragliflozin reduced ELD in renal tubules. Ipragliflozin also reduced the expression levels of GRP78 and CHOP, apoptotic cells, and interstitial fibrosis.
Conclusions: ELD induced kidney injury through ER stress. Ipragliflozin improved the pathogenesis of CKD by reducing ELD and ER stress in NASH-model mice. Our results suggest ipragliflozin has therapeutic effect on CKD in NASH.
Keywords: ER stress; NAFLD; NASH; SGLT2 inhibitor; ectopic fat accumulation; lipotoxicity; steatonephropathy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Ceranowicz P., Warzecha Z., Dembinski A. Peptidyl hormones of endocrine cells origin in the gut—Their discovery and physiological relevance. J. Physiol. Pharmacol. 2015;66:11–27. - PubMed
-
- Guebre-Egziabher F., Alix P.M., Koppe L., Pelletier C.C., Kalbacher E., Fouque D., Soulage C.O. Ectopic lipid accumulation: A potential cause for metabolic disturbances and a contributor to the alteration of kidney function. Biochimie. 2013;95:1971–1979. doi: 10.1016/j.biochi.2013.07.017. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

