Efficient Synthesis of Purine Nucleoside Analogs by a New Trimeric Purine Nucleoside Phosphorylase from Aneurinibacillus migulanus AM007
- PMID: 31888088
- PMCID: PMC6983109
- DOI: 10.3390/molecules25010100
Efficient Synthesis of Purine Nucleoside Analogs by a New Trimeric Purine Nucleoside Phosphorylase from Aneurinibacillus migulanus AM007
Abstract
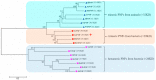
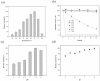
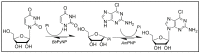
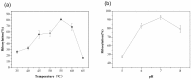
Purine nucleoside phosphorylases (PNPs) are promising biocatalysts for the synthesis of purine nucleoside analogs. Although a number of PNPs have been reported, the development of highly efficient enzymes for industrial applications is still in high demand. Herein, a new trimeric purine nucleoside phosphorylase (AmPNP) from Aneurinibacillus migulanus AM007 was cloned and heterologously expressed in Escherichia coli BL21(DE3). The AmPNP showed good thermostability and a broad range of pH stability. The enzyme was thermostable below 55 °C for 12 h (retaining nearly 100% of its initial activity), and retained nearly 100% of the initial activity in alkaline buffer systems (pH 7.0-9.0) at 60 °C for 2 h. Then, a one-pot, two-enzyme mode of transglycosylation reaction was successfully constructed by combining pyrimidine nucleoside phosphorylase (BbPyNP) derived from Brevibacillus borstelensis LK01 and AmPNP for the production of purine nucleoside analogs. Conversions of 2,6-diaminopurine ribonucleoside (1), 2-amino-6-chloropurine ribonucleoside (2), and 6-thioguanine ribonucleoside (3) synthesized still reached >90% on the higher concentrations of substrates (pentofuranosyl donor: purine base; 20:10 mM) with a low enzyme ratio of BbPyNP: AmPNP (2:20 μg/mL). Thus, the new trimeric AmPNP is a promising biocatalyst for industrial production of purine nucleoside analogs.
Keywords: enzyme ratio; purine nucleoside analogs; thermostability; trimeric nucleoside phosphorylase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Engineering a Bifunctional Fusion Purine/Pyrimidine Nucleoside Phosphorylase for the Production of Nucleoside Analogs.Biomolecules. 2024 Sep 23;14(9):1196. doi: 10.3390/biom14091196. Biomolecules. 2024. PMID: 39334962 Free PMC article.
-
A thermostable pyrimidine nucleoside phosphorylase from Brevibacillus borstelensis LK01 for synthesizing halogenated nucleosides.Biotechnol Lett. 2017 Dec;39(12):1903-1910. doi: 10.1007/s10529-017-2423-1. Epub 2017 Sep 4. Biotechnol Lett. 2017. PMID: 28871515
-
Recombinant purine nucleoside phosphorylases from thermophiles: preparation, properties and activity towards purine and pyrimidine nucleosides.FEBS J. 2013 Mar;280(6):1475-90. doi: 10.1111/febs.12143. Epub 2013 Feb 21. FEBS J. 2013. PMID: 23332162
-
Structural analyses reveal two distinct families of nucleoside phosphorylases.Biochem J. 2002 Jan 1;361(Pt 1):1-25. doi: 10.1042/0264-6021:3610001. Biochem J. 2002. PMID: 11743878 Free PMC article. Review.
-
Chemo-Enzymatic Generation of Highly Fluorescent Nucleoside Analogs Using Purine-Nucleoside Phosphorylase.Biomolecules. 2024 Jun 14;14(6):701. doi: 10.3390/biom14060701. Biomolecules. 2024. PMID: 38927104 Free PMC article. Review.
Cited by
-
Genome-Driven Discovery of Enzymes with Industrial Implications from the Genus Aneurinibacillus.Microorganisms. 2021 Feb 26;9(3):499. doi: 10.3390/microorganisms9030499. Microorganisms. 2021. PMID: 33652876 Free PMC article.
-
Enzymatic Transglycosylation Features in Synthesis of 8-Aza-7-Deazapurine Fleximer Nucleosides by Recombinant E. coli PNP: Synthesis and Structure Determination of Minor Products.Biomolecules. 2024 Jul 4;14(7):798. doi: 10.3390/biom14070798. Biomolecules. 2024. PMID: 39062512 Free PMC article.
-
Magnetic Multi-Enzymatic System for Cladribine Manufacturing.Int J Mol Sci. 2022 Nov 7;23(21):13634. doi: 10.3390/ijms232113634. Int J Mol Sci. 2022. PMID: 36362425 Free PMC article.
-
Engineering Substrate Promiscuity of Nucleoside Phosphorylase Via an Insertions-Deletions Strategy.JACS Au. 2024 Jan 13;4(2):454-464. doi: 10.1021/jacsau.3c00581. eCollection 2024 Feb 26. JACS Au. 2024. PMID: 38425912 Free PMC article.
-
Green Production of Cladribine by Using Immobilized 2'-Deoxyribosyltransferase from Lactobacillus delbrueckii Stabilized through a Double Covalent/Entrapment Technology.Biomolecules. 2021 Apr 29;11(5):657. doi: 10.3390/biom11050657. Biomolecules. 2021. PMID: 33947162 Free PMC article.
References
-
- Tănase C.I., Drăghici C., Hanganu A., Pintilie L., Maganu M., Volobueva A., Sinegubova E., Zarubaev V.V., Neyts J., Jochmans D., et al. New HSV-1 Anti-Viral 1′-Homocarbocyclic Nucleoside Analogs with an Optically Active Substituted Bicyclo[2.2. 1]Heptane Fragment as a Glycoside Moiety. Molecules. 2019;24:2446 - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

