Gene signature characteristic of elevated stromal infiltration and activation is associated with increased risk of hematogenous and lymphatic metastasis in serous ovarian cancer
- PMID: 31888563
- PMCID: PMC6937680
- DOI: 10.1186/s12885-019-6470-y
Gene signature characteristic of elevated stromal infiltration and activation is associated with increased risk of hematogenous and lymphatic metastasis in serous ovarian cancer
Abstract
Background: The clinical significance of hematogenous and lymphatic metastasis in ovarian cancer has been increasingly addressed, as it plays an imperative role in the formation of both intraperitoneal and distant metastases. Our objective is to identify the key molecules and biological processes potentially related to this relatively novel metastatic route in serous ovarian cancer.
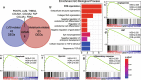
Methods: Since lymphovascular space invasion (LVSI) is considered as the first step of hematogenous and lymphatic dissemination, we developed a gene signature mainly based on the transcriptome profiles with available information on LVSI status in the Cancer Genome Atlas (TCGA) dataset. We then explored the underlying biological rationale and prognostic value of the identified gene signature using multiple public databases.
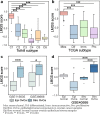
Results: We observe that primary tumors with increased risk of hematogenous and lymphatic metastasis highly express a panel of genes, namely POSTN, LUM, THBS2, COL3A1, COL5A1, COL5A2, FAP1 and FBN1. The identified geneset is characterized by enhanced deposition of extracellular matrix and extensive stromal activation. Mechanistically, both the recruitment and the activation of stromal cells, especially fibroblasts, are closely associated with lymphovascular metastasis. Survival analysis further reveals that the elevated expression of the identified genes correlates to cancer progression and poor prognosis in patients with serous ovarian cancer.
Conclusions: Our findings indicate that tumor stroma supports the hematogenous and lymphatic spread of ovarian cancer, increasing tumor invasiveness and ultimately resulting in worse survival. Thus stroma-targeted therapies may improve the clinical outcomes in combination with cytoreductive surgery and chemotherapy.
Keywords: Cancer-associated fibroblast; Hematogenous and lymphatic metastasis; Lymphovascular space invasion; Ovarian cancer; Tumor stroma.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

