The impact of depression and anxiety treatment on biological aging and metabolic stress: study protocol of the MOod treatment with antidepressants or running (MOTAR) study
- PMID: 31888565
- PMCID: PMC6937704
- DOI: 10.1186/s12888-019-2404-0
The impact of depression and anxiety treatment on biological aging and metabolic stress: study protocol of the MOod treatment with antidepressants or running (MOTAR) study
Abstract
Background: Depressive and anxiety disorders have shown to be associated to premature or advanced biological aging and consequently to adversely impact somatic health. Treatments with antidepressant medication or running therapy are both found to be effective for many but not all patients with mood and anxiety disorders. These interventions may, however, work through different pathophysiological mechanisms and could differ in their impact on biological aging and somatic health. This study protocol describes the design of an unique intervention study that examines whether both treatments are similarly effective in reducing or reversing biological aging (primary outcome), psychiatric status, metabolic stress and neurobiological indicators (secondary outcomes).
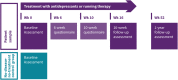
Methods: The MOod Treatment with Antidepressants or Running (MOTAR) study will recruit a total of 160 patients with a current major depressive and/or anxiety disorder in a mental health care setting. Patients will receive a 16-week treatment with either antidepressant medication or running therapy (3 times/week). Patients will undergo the treatment of their preference and a subsample will be randomized (1:1) to overcome preference bias. An additional no-disease-no-treatment group of 60 healthy controls without lifetime psychopathology, will be included as comparison group for primary and secondary outcomes at baseline. Assessments are done at week 0 for patients and controls, and at week 16 and week 52 for patients only, including written questionnaires, a psychiatric and medical examination, blood, urine and saliva collection and a cycle ergometer test, to gather information about biological aging (telomere length and telomerase activity), mental health (depression and anxiety disorder characteristics), general fitness, metabolic stress-related biomarkers (inflammation, metabolic syndrome, cortisol) and genetic determinants. In addition, neurobiological alterations in brain processes will be assessed using structural and functional Magnetic Resonance Imaging (MRI) in a subsample of at least 25 patients per treatment arm and in all controls.
Discussion: This intervention study aims to provide a better understanding of the impact of antidepressant medication and running therapy on biological aging, metabolic stress and neurobiological indicators in patients with depressive and anxiety disorders in order to guide a more personalized medicine treatment.
Trial registration: Trialregister.nl Number of identification: NTR3460, May 2012.
Keywords: Aging; Antidepressant; Anxiety; Cortisol; Depression; Inflammation; Metabolic syndrome; Running therapy; SSRI; Telomerase activity; Telomere length; Treatment; fMRI.
Conflict of interest statement
BP has received (non-related) funding from Jansen Research and Boehringer Ingelheim. All other authors declare that they have no conflicts of interest.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical