Transcranial direct current stimulation in management of pain, mood, functionality, and quality of life in patients undergoing hemodialysis: a study protocol for a double-blind controlled randomized trial
- PMID: 31888699
- PMCID: PMC6937834
- DOI: 10.1186/s13063-019-3769-6
Transcranial direct current stimulation in management of pain, mood, functionality, and quality of life in patients undergoing hemodialysis: a study protocol for a double-blind controlled randomized trial
Abstract
Background: Persistent pain can lead to incapacitation requiring long-term pharmacological treatment. Up to 82% of chronic kidney disease (CKD) patients undergoing hemodialysis (HD) have chronic pain and most do not respond to usual medication. Advances in non-pharmacological treatments are necessary to promote pain relief without side effects and to restore functionality. Transcranial direct current stimulation (tDCS) promises to be a novel, cost-efficient, non-pharmacological treatment for CKD patients with chronic pain. In this study, we hypothesize that tDCS could improve pain, depression, functionality, and quality of life in patients with CKD undergoing HD.
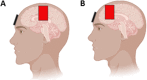
Methods/design: We describe a single-center, parallel-design, double blind randomized, sham-controlled trial. Forty-five subjects with CKD undergoing HD will be randomized to a motor cortex (M1), a dorso lateral prefrontal cortex (DLPFC), or a sham group. A total of ten sessions will be administered to participants over 4 weeks using a monophasic continuous current with an intensity of 2 mA for 20 min. Participants will be evaluated at baseline, immediately after the tenth session, and at 1 week and 4 weeks of follow-up after the intervention. Pain, depression, functionality, and quality of life will be evaluated.
Discussion: The results from this study will provide initial clinical evidence on the efficacy and safety of tDCS in patients with CKD undergoing HD.
Trial registration: Brazilian Clinical Trials Registry/Registro Brasileiro de Ensaios Clínicos (ensaiosclinicos.gov.br), 1111-1216-0137. Registered on 20 June 2018.
Keywords: Brain stimulation; Depression; Kidney diseases; Pain; Quality of life.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

