Microglia in the developing retina
- PMID: 31888774
- PMCID: PMC6938006
- DOI: 10.1186/s13064-019-0137-x
Microglia in the developing retina
Abstract
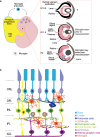
Microglia are increasingly shown to be key players in neuron development and synapse connectivity. However, the underlying mechanisms by which microglia regulate neuron function remain poorly understood in part because such analysis is challenging in the brain where neurons and synapses are intermingled and connectivity is only beginning to be mapped. Here, we discuss the features and function of microglia in the ordered mammalian retina where the laminar organization of neurons and synapses facilitates such molecular studies. We discuss microglia origins and consider the evidence for molecularly distinct microglia subpopulations and their potential for differential roles with a particular focus on the early stages of retina development. We then review the models and methods used for the study of these cells and discuss emerging data that link retina microglia to the genesis and survival of particular retina cell subtypes. We also highlight potential roles for microglia in shaping the development and organization of the vasculature and discuss cellular and molecular mechanisms involved in this process. Such insights may help resolve the mechanisms by which retinal microglia impact visual function and help guide studies of related features in brain development and disease.
Keywords: Brain; Depletion models; Development; Microglia; Retina; Synapse.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

