Characterization of potential biomarkers of reactogenicity of licensed antiviral vaccines: randomized controlled clinical trials conducted by the BIOVACSAFE consortium
- PMID: 31889148
- PMCID: PMC6937244
- DOI: 10.1038/s41598-019-56994-8
Characterization of potential biomarkers of reactogenicity of licensed antiviral vaccines: randomized controlled clinical trials conducted by the BIOVACSAFE consortium
Abstract
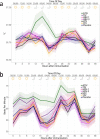
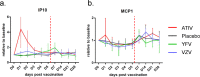
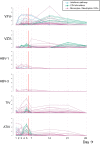
Biomarkers predictive of inflammatory events post-vaccination could accelerate vaccine development. Within the BIOVACSAFE framework, we conducted three identically designed, placebo-controlled inpatient/outpatient clinical studies (NCT01765413/NCT01771354/NCT01771367). Six antiviral vaccination strategies were evaluated to generate training data-sets of pre-/post-vaccination vital signs, blood changes and whole-blood gene transcripts, and to identify putative biomarkers of early inflammation/reactogenicity that could guide the design of subsequent focused confirmatory studies. Healthy adults (N = 123; 20-21/group) received one immunization at Day (D)0. Alum-adjuvanted hepatitis B vaccine elicited vital signs and inflammatory (CRP/innate cells) responses that were similar between primed/naive vaccinees, and low-level gene responses. MF59-adjuvanted trivalent influenza vaccine (ATIV) induced distinct physiological (temperature/heart rate/reactogenicity) response-patterns not seen with non-adjuvanted TIV or with the other vaccines. ATIV also elicited robust early (D1) activation of IFN-related genes (associated with serum IP-10 levels) and innate-cell-related genes, and changes in monocyte/neutrophil/lymphocyte counts, while TIV elicited similar but lower responses. Due to viral replication kinetics, innate gene activation by live yellow-fever or varicella-zoster virus (YFV/VZV) vaccines was more suspended, with early IFN-associated responses in naïve YFV-vaccine recipients but not in primed VZV-vaccine recipients. Inflammatory responses (physiological/serum markers, innate-signaling transcripts) are therefore a function of the vaccine type/composition and presence/absence of immune memory. The data reported here have guided the design of confirmatory Phase IV trials using ATIV to provide tools to identify inflammatory or reactogenicity biomarkers.
Conflict of interest statement
P.D., R.v.d.B. and G.D.G. are employees of the GSK group of companies, and report receiving restricted shares of the company. K.K. is employee of Sanofi Pasteur. I.J. is employee of deCode genetics/Amgen Inc. A.M. and B.B. are receiving royalties for reagents to measure PTX3 levels. Other authors report no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

