AGI-134: a fully synthetic α-Gal glycolipid that converts tumors into in situ autologous vaccines, induces anti-tumor immunity and is synergistic with an anti-PD-1 antibody in mouse melanoma models
- PMID: 31889898
- PMCID: PMC6923872
- DOI: 10.1186/s12935-019-1059-8
AGI-134: a fully synthetic α-Gal glycolipid that converts tumors into in situ autologous vaccines, induces anti-tumor immunity and is synergistic with an anti-PD-1 antibody in mouse melanoma models
Abstract
Background: Treatments that generate T cell-mediated immunity to a patient's unique neoantigens are the current holy grail of cancer immunotherapy. In particular, treatments that do not require cumbersome and individualized ex vivo processing or manufacturing processes are especially sought after. Here we report that AGI-134, a glycolipid-like small molecule, can be used for coating tumor cells with the xenoantigen Galα1-3Galβ1-4GlcNAc (α-Gal) in situ leading to opsonization with pre-existing natural anti-α-Gal antibodies (in short anti-Gal), which triggers immune cascades resulting in T cell mediated anti-tumor immunity.
Methods: Various immunological effects of coating tumor cells with α-Gal via AGI-134 in vitro were measured by flow cytometry: (1) opsonization with anti-Gal and complement, (2) antibody-dependent cell-mediated cytotoxicity (ADCC) by NK cells, and (3) phagocytosis and antigen cross-presentation by antigen presenting cells (APCs). A viability kit was used to test AGI-134 mediated complement dependent cytotoxicity (CDC) in cancer cells. The anti-tumoral activity of AGI-134 alone or in combination with an anti-programmed death-1 (anti-PD-1) antibody was tested in melanoma models in anti-Gal expressing galactosyltransferase knockout (α1,3GT-/-) mice. CDC and phagocytosis data were analyzed by one-way ANOVA, ADCC results by paired t-test, distal tumor growth by Mantel-Cox test, C5a data by Mann-Whitney test, and single tumor regression by repeated measures analysis.
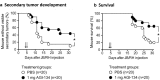
Results: In vitro, α-Gal labelling of tumor cells via AGI-134 incorporation into the cell membrane leads to anti-Gal binding and complement activation. Through the effects of complement and ADCC, tumor cells are lysed and tumor antigen uptake by APCs increased. Antigen associated with lysed cells is cross-presented by CD8α+ dendritic cells leading to activation of antigen-specific CD8+ T cells. In B16-F10 or JB/RH melanoma models in α1,3GT-/- mice, intratumoral AGI-134 administration leads to primary tumor regression and has a robust abscopal effect, i.e., it protects from the development of distal, uninjected lesions. Combinations of AGI-134 and anti-PD-1 antibody shows a synergistic benefit in protection from secondary tumor growth.
Conclusions: We have identified AGI-134 as an immunotherapeutic drug candidate, which could be an excellent combination partner for anti-PD-1 therapy, by facilitating tumor antigen processing and increasing the repertoire of tumor-specific T cells prior to anti-PD-1 treatment.
Keywords: AGI-134; Abscopal effect; Cancer vaccine; Checkpoint inhibition; Immunotherapy; Intratumoral injection; Melanoma; alpha-Gal; anti-Gal; anti-PD-1.
© The Author(s) 2019.
Conflict of interest statement
Competing interestsAll of the authors are or were employees, have or had executive roles and/or are consultants to, and may have stocks or shares of Agalimmune Ltd. or BioLineRx, and may hold patents related to the described work. Several of the authors are or were involved in efforts to develop AGI-134 as cancer immunotherapy. AGI-134 is currently in Phase 1/2a clinical trials in the United Kingdom and Israel with BioLineRx as sponsor.
Figures





Similar articles
-
Increased immunogenicity of tumor vaccines complexed with anti-Gal: studies in knockout mice for alpha1,3galactosyltransferase.Cancer Res. 1999 Jul 15;59(14):3417-23. Cancer Res. 1999. PMID: 10416604
-
Self-Tumor Antigens in Solid Tumors Turned into Vaccines by α-gal Micelle Immunotherapy.Pharmaceutics. 2024 Sep 27;16(10):1263. doi: 10.3390/pharmaceutics16101263. Pharmaceutics. 2024. PMID: 39458595 Free PMC article. Review.
-
Autologous tumor vaccines processed to express alpha-gal epitopes: a practical approach to immunotherapy in cancer.Cancer Immunol Immunother. 2004 Nov;53(11):935-45. doi: 10.1007/s00262-004-0524-x. Epub 2004 Jun 16. Cancer Immunol Immunother. 2004. PMID: 15205919 Free PMC article. Review.
-
The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy.Immunol Cell Biol. 2005 Dec;83(6):674-86. doi: 10.1111/j.1440-1711.2005.01366.x. Immunol Cell Biol. 2005. PMID: 16266320 Review.
-
Intratumoral injection of alpha-gal glycolipids induces xenograft-like destruction and conversion of lesions into endogenous vaccines.J Immunol. 2007 Apr 1;178(7):4676-87. doi: 10.4049/jimmunol.178.7.4676. J Immunol. 2007. PMID: 17372027
Cited by
-
Neoantigens and their potential applications in tumor immunotherapy.Oncol Lett. 2022 Mar;23(3):88. doi: 10.3892/ol.2022.13208. Epub 2022 Jan 21. Oncol Lett. 2022. PMID: 35126730 Free PMC article. Review.
-
Antibody production and tolerance to the α-gal epitope as models for understanding and preventing the immune response to incompatible ABO carbohydrate antigens and for α-gal therapies.Front Mol Biosci. 2023 Jun 28;10:1209974. doi: 10.3389/fmolb.2023.1209974. eCollection 2023. Front Mol Biosci. 2023. PMID: 37449060 Free PMC article. Review.
-
Intratumoural immunotherapies for unresectable and metastatic melanoma: current status and future perspectives.Br J Cancer. 2020 Sep;123(6):885-897. doi: 10.1038/s41416-020-0994-4. Epub 2020 Jul 27. Br J Cancer. 2020. PMID: 32713938 Free PMC article. Review.
-
Secreted phosphoprotein 1 as a potential prognostic and immunotherapy biomarker in multiple human cancers.Bioengineered. 2022 Feb;13(2):3221-3239. doi: 10.1080/21655979.2021.2020391. Bioengineered. 2022. PMID: 35067176 Free PMC article.
-
Live unattenuated vaccines for controlling viral diseases, including COVID-19.J Med Virol. 2021 Apr;93(4):1943-1949. doi: 10.1002/jmv.26453. Epub 2020 Sep 29. J Med Virol. 2021. PMID: 32833258 Free PMC article. Review.
References
-
- Galili U, et al. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263(33):17755–17762. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

