Physical properties of secondary photochemical aerosol from OH oxidation of a cyclic siloxane
- PMID: 31889955
- PMCID: PMC6936766
- DOI: 10.5194/acp-19-1649-2019
Physical properties of secondary photochemical aerosol from OH oxidation of a cyclic siloxane
Abstract
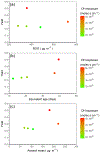
Cyclic volatile methyl siloxanes (cVMS) are high-production chemicals present in many personal care products. They are volatile, hydrophobic, and relatively long-lived due to slow oxidation kinetics. Evidence from chamber and ambient studies indicates that oxidation products may be found in the condensed aerosol phase. In this work, we use an oxidation flow reactor to produce ~ 100 μgm-3 of organosilicon aerosol from OH oxidation of decamethyl-cyclopentasiloxane (D5) with aerosol mass fractions (i.e., yields) of 0.2-0.5. The aerosols were assessed for concentration, size distribution, morphology, sensitivity to seed aerosol, hygroscopicity, volatility and chemical composition through a combination of aerosol size distribution measurement, tandem differential mobility analysis, and electron microscopy. Similar aerosols were produced when vapor from solid antiperspirant was used as the reaction precursor. Aerosol yield was sensitive to chamber OH and to seed aerosol, suggesting sensitivity of lower-volatility species and recovered yields to oxidation conditions and chamber operation. The D5 oxidation aerosol products were relatively non-hygroscopic, with an average hygroscopicity kappa of ~ 0.01, and nearly non-volatile up to 190 °C temperature. Parameters for exploratory treatment as a semi-volatile organic aerosol in atmospheric models are provided.
Conflict of interest statement
Competing interests. The authors declare that they have no conflict of interest.
Figures






References
-
- Atkinson R: Kinetics of the Gas-Phase Reactions of a Series of Organosilicon Compounds with OH and NO3 Radicals and O3 at 297 + / − 2 K, Environ. Sci. Technol, 25, 863–866, 10.1021/es00017a005, 1991. - DOI
-
- Brooke D, Crookes M, Gray D, and Robertson S: Environmental Risk Assessment Report: Decamethylcyclopentasiloxane, Environment Agency of England and Wales, Bristol, UK, ISBN 978–1-84911–029-7, 223 pp., 2009a.
-
- Brooke D, Crookes M, Gray D, and Robertson S: Environmental Risk Assessment Report: Dodecamethylcyclohexasiloxane, Environment Agency of England and Wales, Bristol, UK, ISBN 978-1-84911-030-3, 108 pp. 2009b.
-
- Brooke D, Crookes M, Gray D, and Robertson S: Environmental Risk Assessment Report: Octamethylcyclotetrasiloxane, Environment Agency of England and Wales, Bristol, UK, ISBN 978-1-84911-031-0, 201 pp., 2009c.
Grants and funding
LinkOut - more resources
Full Text Sources
