Mandible and iliac osteoblasts exhibit different Wnt signaling responses to LMHF vibration
- PMID: 31890493
- PMCID: PMC6924195
- DOI: 10.1016/j.jobcr.2019.09.005
Mandible and iliac osteoblasts exhibit different Wnt signaling responses to LMHF vibration
Abstract
Objective: The jaw bones and long bones have distinct developmental origins and respond differently to mechanical stimuli. This study aimed to compare the Wnt signaling responses of human mandible osteoblasts and long bone osteoblasts to low-magnitude, high-frequency (LMHF) mechanical vibration in vitro.
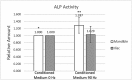
Methods: Primary human osteoblast cultures were prepared from mandibular bone (n = 3) and iliac bone (n = 3) specimens (six individuals). Osteoblast cell lines were subjected to vibration (0, 30, 60, 90, or 120 Hz) for 30 min. After 24 h, cells were vibrated for 30 min again, then harvested immediately to quantify Wnt10b, Wnt5a and runt-related transcription factor 2 (RUNX2) mRNA expression, β-catenin protein expression and alkaline phosphatase (ALP) activity.
Results: Mandible and iliac osteoblasts responded differently to LMHF vibration: Wnt10b mRNA was upregulated by the frequency range tested; Wnt5a, β-catenin protein expression and RUNX2 mRNA expression were not altered. Furthermore, vibration upregulated ALP activity in mandible osteoblasts, but not in iliac osteoblasts.
Conclusions: This study demonstrates mandible osteoblasts and long bone osteoblasts respond differently to LMHF mechanical vibration in terms of Wnt signaling expression and ALP activity. Therefore, the effects of whole-body vibration on the long bones cannot be generalized to the jaw bones. Furthermore, osteoblast-like cells mediate the cellular responses to vibration, at least in part, by secreting extracellular signaling molecules.
Keywords: ALP, alkaline phosphatase; Alkaline phosphatase; Catenin; Human; LMHF vibration, low-magnitude, high-frequency vibration; Mandible; Osteoblast; RUNX2, runt-related transcription factor 2; Vibration; hIOBs, human iliac crest-derived mature osteoblast-like cells; hMOBs, human mandible-derived osteoblast-like cells.
© 2019 Craniofacial Research Foundation. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors have no potential conflicts of interest with respect to the authorship and/or publication of this article to declare.
Figures



References
-
- Akintoye S.O., Lam T., Shi S., Brahim J., Collins M.T., Robey P.G. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38(6):758–768. - PubMed
-
- Suttapreyasri S., Koontongkaew S., Phongdara A., Leggat U. Expression of bone morphogenetic proteins in normal human intramembranous and endochondral bones. Int J Oral Maxillofac Surg. 2006;35(5):444–452. - PubMed
-
- Rawlinson S.C., Mosley J.R., Suswillo R.F., Pitsillides A.A., Lanyon L.E. Calvarial and limb bone cells in organ and monolayer culture do not show the same early responses to dynamic mechanical strain. J Bone Miner Res. 1995;10(8):1225–1232. - PubMed
-
- Tripuwabhrut P., Mustafa M., Gjerde C.G., Brudvik P., Mustafa K. Effect of compressive force on human osteoblast-like cells and bone remodelling: an in vitro study. Arch Oral Biol. 2013;58(7):826–836. - PubMed
LinkOut - more resources
Full Text Sources

