Production of tetra-methylpyrazine using engineered Corynebacterium glutamicum
- PMID: 31890587
- PMCID: PMC6926172
- DOI: 10.1016/j.mec.2019.e00115
Production of tetra-methylpyrazine using engineered Corynebacterium glutamicum
Abstract
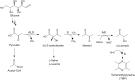
Corynebacterium glutamicum ATCC 13032 is an established and industrially-relevant microbial host that has been utilized for the expression of many desirable bioproducts. Tetra-methylpyrazine (TMP) is a naturally occurring alkylpyrazine with broad applications spanning fragrances to resins. We identified an engineered strain of C. glutamicum which produces 5 g/L TMP and separately, a strain which can co-produce both TMP and the biofuel compound isopentenol. Ionic liquids also stimulate TMP production in engineered strains. Using a fed batch-mode feeding strategy, ionic liquid stimulated strains produced 2.2 g/L of tetra-methylpyrazine. We show that feedback from a specific heterologous gene pathway on host physiology leads to acetoin accumulation and the production of TMP.
Keywords: 2,3,5,6-Tetra-methylpyrazine; Alkaloids; Bioreactor; Corynebacterium glutamicum; Isopentenol; Terpene.
© 2019 The Authors.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures






References
-
- Baral N.R., Kavvada O., Mendez-Perez D., Mukhopadhyay A., Lee T.S., Simmons B.A., Scown C.D. Techno-economic analysis and life-cycle greenhouse gas mitigation cost of five routes to bio-jet fuel blendstocks. Energy Environ. Sci. 2019;12:807–824.
-
- Baral N.R., Sundstrom E.R., Das L., Gladden J., Eudes A., Mortimer J.C., Singer S.W., Mukhopadhyay A., Scown C.D. Approaches for more efficient biological conversion of lignocellulosic feedstocks to biofuels and bioproducts. ACS Sustain. Chem. Eng. 2019;7(10):9062–9079.
-
- Deng T., Tao S., Luo D., Qin P. 2006. China Patent CN1546474A. Method for Preparing Tetramethyl Pyrazine.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

