GBM-Targeted oHSV Armed with Matrix Metalloproteinase 9 Enhances Anti-tumor Activity and Animal Survival
- PMID: 31890868
- PMCID: PMC6926261
- DOI: 10.1016/j.omto.2019.10.005
GBM-Targeted oHSV Armed with Matrix Metalloproteinase 9 Enhances Anti-tumor Activity and Animal Survival
Abstract
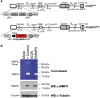
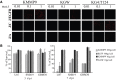
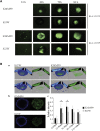
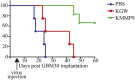
The use of mutant strains of oncolytic herpes simplex virus (oHSV) in early-phase human clinical trials for the treatment of glioblastoma multiforme (GBM) has proven safe, but limited efficacy suggests that more potent vector designs are required for effective GBM therapy. Inadequate vector performance may derive from poor intratumoral vector replication and limited spread to uninfected cells. Vector replication may be impaired by mutagenesis strategies to achieve vector safety, and intratumoral virus spread may be hampered by vector entrapment in the tumor-specific extracellular matrix (ECM) that in GBM is composed primarily of type IV collagen. In this report, we armed our previously described epidermal growth factor receptor (EGFR)vIII-targeted, neuronal microRNA-sensitive oHSV with a matrix metalloproteinase (MMP9) to improve intratumoral vector distribution. We show that vector-expressed MMP9 enhanced therapeutic efficacy and long-term animal survival in a GBM xenograft model.
Keywords: Glioblastoma multiforme = GBM; OVs derived from herpes simplex virus = oHSV; Oncolytic vectors = OVs; extracellular matrix = ECM; matrix metalloproteinase 9 = MMP9.
© 2019 The Authors.
Figures
Similar articles
-
Stem cells loaded with multimechanistic oncolytic herpes simplex virus variants for brain tumor therapy.J Natl Cancer Inst. 2014 May 16;106(6):dju090. doi: 10.1093/jnci/dju090. Print 2014 Jun. J Natl Cancer Inst. 2014. PMID: 24838834
-
Blockade of transforming growth factor-β signaling enhances oncolytic herpes simplex virus efficacy in patient-derived recurrent glioblastoma models.Int J Cancer. 2017 Dec 1;141(11):2348-2358. doi: 10.1002/ijc.30929. Epub 2017 Aug 26. Int J Cancer. 2017. PMID: 28801914 Free PMC article.
-
Oncolytic herpes simplex virus-based strategies: toward a breakthrough in glioblastoma therapy.Front Microbiol. 2014 Jun 20;5:303. doi: 10.3389/fmicb.2014.00303. eCollection 2014. Front Microbiol. 2014. PMID: 24999342 Free PMC article. Review.
-
The Current State of Oncolytic Herpes Simplex Virus for Glioblastoma Treatment.Oncolytic Virother. 2021 Feb 24;10:1-27. doi: 10.2147/OV.S268426. eCollection 2021. Oncolytic Virother. 2021. PMID: 33659221 Free PMC article. Review.
-
Combination of oncolytic herpes simplex viruses armed with angiostatin and IL-12 enhances antitumor efficacy in human glioblastoma models.Neoplasia. 2013 Jun;15(6):591-9. doi: 10.1593/neo.13158. Neoplasia. 2013. PMID: 23730207 Free PMC article.
Cited by
-
Glioblastoma microenvironment and its reprogramming by oncolytic virotherapy.Front Cell Neurosci. 2022 Sep 9;16:819363. doi: 10.3389/fncel.2022.819363. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36159398 Free PMC article. Review.
-
Herpes Simplex Virus Oncolytic Immunovirotherapy: The Blossoming Branch of Multimodal Therapy.Int J Mol Sci. 2020 Nov 5;21(21):8310. doi: 10.3390/ijms21218310. Int J Mol Sci. 2020. PMID: 33167582 Free PMC article. Review.
-
Recent Developments in Glioblastoma Therapy: Oncolytic Viruses and Emerging Future Strategies.Viruses. 2023 Feb 16;15(2):547. doi: 10.3390/v15020547. Viruses. 2023. PMID: 36851761 Free PMC article. Review.
-
Unmasking the potential: mechanisms of neuroinflammatory modulation by oncolytic viruses in glioblastoma.Explor Target Antitumor Ther. 2025 Feb 24;6:1002294. doi: 10.37349/etat.2025.1002294. eCollection 2025. Explor Target Antitumor Ther. 2025. PMID: 40061139 Free PMC article. Review.
-
Tutorial: design, production and testing of oncolytic viruses for cancer immunotherapy.Nat Protoc. 2024 Sep;19(9):2540-2570. doi: 10.1038/s41596-024-00985-1. Epub 2024 May 20. Nat Protoc. 2024. PMID: 38769145 Review.
References
-
- Mohyeldin A., Chiocca E.A. Gene and viral therapy for glioblastoma: a review of clinical trials and future directions. Cancer J. 2012;18:82–88. - PubMed
-
- Yin A.A., Cheng J.X., Zhang X., Liu B.L. The treatment of glioblastomas: a systematic update on clinical phase III trials. Crit. Rev. Oncol. Hematol. 2013;87:265–282. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

