Phase III, placebo-controlled, randomized, double-blind trial of tableted, therapeutic TB vaccine (V7) containing heat-killed M. vaccae administered daily for one month
- PMID: 31890902
- PMCID: PMC6933248
- DOI: 10.1016/j.jctube.2019.100141
Phase III, placebo-controlled, randomized, double-blind trial of tableted, therapeutic TB vaccine (V7) containing heat-killed M. vaccae administered daily for one month
Abstract
Objective: Immunotherapy of tuberculosis (TB) to shorten treatment duration represents an unmet medical need. Orally delivered, tableted TB vaccine (V7) containing heat-killed Mycobacterium vaccae (NCTC 11659) has been demonstrated in prior clinical studies to be safe and fast-acting immune adjunct.
Methods: The outcome of Phase III trial of V7 containing 10 µg of hydrolyzed M. vaccae was evaluated in 152 patients randomized at 2:1 ratio: V7 (N = 100), placebo (N = 52). Both arms received conventional 1st or 2nd line TB drugs co-administered with daily pill of V7 or placebo.
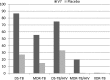
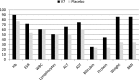
Results: After one month mycobacterial clearance was observed in 68% (P < 0.0001) and 23.1% (P = 0.04) of patients on V7 and placebo. Stratified conversion rates in V7 recipients with drug-sensitive and multidrug-resistant TB were 86.7% and 55.6% vs 27.2% and 15% in placebo. Patients on V7 gained on average 2.4 kg (P < 0.0001) vs 0.3 kg (P = 0.18) in placebo. Improvements in hemoglobin levels, erythrocyte sedimentation rate and leukocyte counts were significantly better than in controls. Liver function tests revealed that V7 can prevent chemotherapy-induced hepatic damage.
Conclusion: Oral M. vaccae is safe, can overcome TB-associated weight loss and inflammation, reduce hepatotoxicity of TB drugs, improve sputum conversion three-fold OR 3.15; 95%CI (2.3,4.6), and cut treatment length by at least six-fold. Longer follow-up studies might be needed to further substantiate our findings (Clinicaltrials.gov: NCT01977768).
Keywords: Immunotherapy; MDR; Mycobacterium vaccae; Therapeutic vaccine; XDR.
© 2019 The Author(s).
Conflict of interest statement
ASB, VB, MT, VK, VJ, AR and AIB are officers of Immunitor and affiliated companies. Late John Stanford and his colleague and spouse Cynthia Stanford are founders and owners of BioEos company. Remaining authors declare no conflict of interest.
Figures
References
-
- Dubrovina I, Miskinis K, Lyepshina S, Yann Y, Hoffmann H, Zaleskis R, Nunn P, Zignol M. Drug-resistant tuberculosis and HIV in Ukraine: a threatening convergence of two epidemics? Int J Tuberc Lung Dis. 2008;12(7):756–762. - PubMed
-
- WHO Country Report: TB situation in Ukraine 2017 https://extranet.who.int/sree/Reports?oP=Replet&name=/WHO_HQ_Reports/G2/....
-
- Pavlenko E, Barbova A, Hovhannesyan A, Tsenilova Z, Slavuckij A, Shcherbak-Verlan B, Zhurilo A, Vitek E, Skenders G, Sela I, Cabibbe AM, Cirillo DM, de Colombani P, Dara M, Dean A, Zignol M, Dadu A. Alarming levels of multidrug-resistant tuberculosis in Ukraine: results from the first national survey. Int J Tuberc Lung Dis. 2018;22(2):197–205. - PubMed
-
- WPRO Tuberculosishttp://www.wpro.who.int/mongolia/topics/tuberculosis/en/.
-
- Buyankhishig B, Naranbat N, Mitarai S, Rieder HL. Nationwide survey of anti-tuberculosis drug resistance in Mongolia. Int J Tuberc Lung Dis. 2011;15(9):1201–1205. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

