Adaptation within embryonic and neonatal heart environment reveals alternative fates for adult c-kit+ cardiac interstitial cells
- PMID: 31891237
- PMCID: PMC7180292
- DOI: 10.1002/sctm.19-0277
Adaptation within embryonic and neonatal heart environment reveals alternative fates for adult c-kit+ cardiac interstitial cells
Abstract
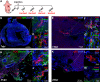
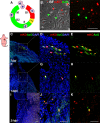
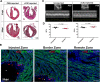
Cardiac interstitial cells (CICs) perform essential roles in myocardial biology through preservation of homeostasis as well as response to injury or stress. Studies of murine CIC biology reveal remarkable plasticity in terms of transcriptional reprogramming and ploidy state with important implications for function. Despite over a decade of characterization and in vivo utilization of adult c-Kit+ CIC (cCIC), adaptability and functional responses upon delivery to adult mammalian hearts remain poorly understood. Limitations of characterizing cCIC biology following in vitro expansion and adoptive transfer into the adult heart were circumvented by delivery of the donated cells into early cardiogenic environments of embryonic, fetal, and early postnatal developing hearts. These three developmental stages were permissive for retention and persistence, enabling phenotypic evaluation of in vitro expanded cCICs after delivery as well as tissue response following introduction to the host environment. Embryonic blastocyst environment prompted cCIC integration into trophectoderm as well as persistence in amniochorionic membrane. Delivery to fetal myocardium yielded cCIC perivascular localization with fibroblast-like phenotype, similar to cCICs introduced to postnatal P3 heart with persistent cell cycle activity for up to 4 weeks. Fibroblast-like phenotype of exogenously transferred cCICs in fetal and postnatal cardiogenic environments is consistent with inability to contribute directly toward cardiogenesis and lack of functional integration with host myocardium. In contrast, cCICs incorporation into extra-embryonic membranes is consistent with fate of polyploid cells in blastocysts. These findings provide insight into cCIC biology, their inherent predisposition toward fibroblast fates in cardiogenic environments, and remarkable participation in extra-embryonic tissue formation.
Keywords: adaptation; cardiac; cell culture; heart; interstitial cell.
© 2019 The Authors. Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Conflict of interest statement
M.A.S. is a founding member of CardioCreate, Inc. The remaining authors declared no potential conflicts of interest.
Figures



References
-
- Gude NA, Broughton KM, Firouzi F, Sussman MA. Cardiac ageing: extrinsic and intrinsic factors in cellular renewal and senescence. Nat Rev Cardiol. 2018;15:523‐542. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

