Oral roxadustat three times weekly in ESA-naïve and ESA-converted patients with anemia of chronic kidney disease on hemodialysis: Results from two phase 3 studies
- PMID: 31891449
- PMCID: PMC7687179
- DOI: 10.1111/1744-9987.13468
Oral roxadustat three times weekly in ESA-naïve and ESA-converted patients with anemia of chronic kidney disease on hemodialysis: Results from two phase 3 studies
Abstract
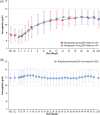
Roxadustat is a hypoxia-inducible factor prolyl hydroxylase inhibitor approved in China for anemia of dialysis-dependent chronic kidney disease (CKD). Japanese hemodialysis patients with anemia of CKD previously naïve to, or converted from, erythropoiesis-stimulating agents (ESAs) were enrolled in two open-label, noncomparative studies of titrated oral roxadustat administered three times weekly. ESA-naïve patients (n = 75) were randomized to roxadustat (initial dose, 50 or 70 mg) for 24 weeks; ESA-converted patients (n = 164) were assigned to roxadustat (initial dose, 70 or 100 mg based on prior ESA dose) for 52 weeks. Efficacy outcomes included average hemoglobin (Hb, weeks 18-24 or 46-52), change of Hb from baseline to weeks 18 to 24 (ΔHb18-24 ) or weeks 46 to 52 (ΔHb46-52 ), and maintenance rate (proportion of patients who achieved average Hb of 10.0-12.0 g/dL for weeks 18-24 or weeks 46-52). Treatment-emergent adverse events (TEAEs) were monitored. Mean (SD) Hb was 10.93 (0.79) g/dL (weeks 18-24) (ESA-Naïve Study), and 10.93 (0.69; weeks 18-24) g/dL and 11.11 (0.67; weeks 46-52) g/dL (ESA-Converted Study). Mean (SD) ΔHb18-24 was 2.26 (1.02) g/dL (ESA-Naïve Study) and -0.03 (0.90) g/dL (ESA-Converted Study); mean (SD) ΔHb46-52 was 0.12 (0.83) g/dL (ESA-Converted Study). The overall maintenance rate was 73.0% (54/74) (ESA-Naïve Study) (weeks 18-24), and 79.1% (129/163; weeks 18-24) and 71.2% (116/163; weeks 46-52) (ESA-Converted Study). Nasopharyngitis was the most common TEAE. Two deaths, considered unrelated to roxadustat, occurred in the ESA-Converted Study. Roxadustat effectively corrected and maintained Hb, regardless of previous ESA treatment, in Japanese anemic CKD patients on hemodialysis.
Keywords: anemia; chronic kidney disease; erythropoiesis-stimulating agent; hemodialysis; roxadustat.
© 2019 The Authors. Therapeutic Apheresis and Dialysis published by John Wiley & Sons Australia, Ltd on behalf of International Society for Apheresis, Japanese Society for Apheresis, and Japanese Society for Dialysis Therapy.
Conflict of interest statement
Tadao Akizawa reports personal fees from Astellas during the conduct of the study, and personal fees from: Bayer Yakuhin, Ltd.; Japan Tobacco, Inc.; GlaxoSmithKline; Kissei Pharmaceutical Co., Ltd.; Chugai Pharmaceutical Co. Ltd; Ono Pharmaceutical Co. Ltd.; Fuso Pharmaceutical Industries, Ltd.; Nipro Corporation; Kyowa Kirin; Otsuka; Sanwa; and Torii Pharmaceutical Co., Ltd. outside of the submitted work. Michael Reusch is an employee of Astellas Pharma Europe B.V. Mai Ueno and Takanori Shiga own stock in Astellas Pharma, Inc. and are employees of Astellas Pharma, Inc.
Figures
References
-
- Locatelli F, Fishbane S, Block GA, Macdougall IC. Targeting hypoxia‐inducible factors for the treatment of anemia in chronic kidney disease patients. Am J Nephrol. 2017;45(3):187–199. - PubMed
-
- Del Vecchio L, Locatelli F. An overview on safety issues related to erythropoiesis‐stimulating agents for the treatment of anaemia in patients with chronic kidney disease. Expert Opin Drug Saf. 2016;15(8):1021–1030. - PubMed
-
- KDIGO . KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2(4):279–335.
-
- Akizawa T, Okumura H, Alexandre AF, Fukushima A, Kiyabu G, Dorey J. Burden of anemia in chronic kidney disease patients in Japan: A literature review. Ther Apheresis Dial. 2018;22(5):444–456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

