Endothelial nitric oxide synthase activation is required for heparin receptor effects on vascular smooth muscle cells
- PMID: 31891520
- PMCID: PMC7099515
- DOI: 10.1152/ajpcell.00284.2018
Endothelial nitric oxide synthase activation is required for heparin receptor effects on vascular smooth muscle cells
Abstract
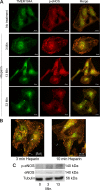
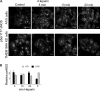
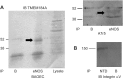
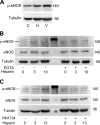
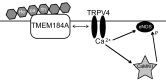
Published studies indicate that TMEM184A is a heparin receptor that interacts with and transduces stimulation from heparin in vascular cells. Previous studies have indicated that heparin increases endothelial nitric oxide synthase (eNOS) activity in bovine endothelial cells. However, the precise mechanism remains unknown. In this study, we investigated the impact of heparin treatment and TMEM184A on eNOS's activation and the role of eNOS in heparin signaling in the cloned A7r5 rat vascular smooth muscle cell line and confirmed results in endothelial cells. We employed a combination of TMEM184A knockdown A7r5 cells along with transient eNOS knockdown and enzyme inhibitor strategies. The results indicate that heparin induces phosphorylation of eNOS. eNOS can be immunoprecipitated with TMEM184A and is internalized to the perinuclear region in a TMEM184A-dependent manner in response to heparin. We also examined how heparin treatment leads to phosphorylation of eNOS and confirmed that TMEM184A and Ca2+ were required to mediate heparin-elicited eNOS phosphorylation. Evidence supporting the involvement of transient receptor potential cation channel subfamily V member 4 with TMEM184A in this eNOS activation process is also presented.
Keywords: eNOS; heparin; vascular smooth muscle.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




Similar articles
-
Transmembrane Protein 184A Is a Receptor Required for Vascular Smooth Muscle Cell Responses to Heparin.J Biol Chem. 2016 Mar 4;291(10):5326-41. doi: 10.1074/jbc.M115.681122. Epub 2016 Jan 14. J Biol Chem. 2016. PMID: 26769966 Free PMC article.
-
Ambient pressure upregulates nitric oxide synthase in a phosphorylated-extracellular regulated kinase- and protein kinase C-dependent manner.J Vasc Surg. 2006 Nov;44(5):1076-84. doi: 10.1016/j.jvs.2006.06.033. J Vasc Surg. 2006. PMID: 17098545
-
Mechanism of endothelial nitric oxide synthase phosphorylation and activation by thrombin.Hypertension. 2007 Mar;49(3):577-83. doi: 10.1161/01.HYP.0000255954.80025.34. Epub 2007 Jan 8. Hypertension. 2007. PMID: 17210830
-
Heparin Decreases in Tumor Necrosis Factor α (TNFα)-induced Endothelial Stress Responses Require Transmembrane Protein 184A and Induction of Dual Specificity Phosphatase 1.J Biol Chem. 2016 Mar 4;291(10):5342-54. doi: 10.1074/jbc.M115.681288. Epub 2016 Jan 14. J Biol Chem. 2016. PMID: 26769965 Free PMC article.
-
The Ever-Expanding Influence of the Endothelial Nitric Oxide Synthase.Basic Clin Pharmacol Toxicol. 2025 May;136(5):e70029. doi: 10.1111/bcpt.70029. Basic Clin Pharmacol Toxicol. 2025. PMID: 40150952 Free PMC article. Review.
Cited by
-
Involvement of transmembrane protein 184a during angiogenesis in zebrafish embryos.Front Physiol. 2022 Sep 2;13:845407. doi: 10.3389/fphys.2022.845407. eCollection 2022. Front Physiol. 2022. PMID: 36117693 Free PMC article.
-
Effect of blood flow restriction training on health promotion in middle-aged and elderly women: a systematic review and meta-analysis.Front Physiol. 2024 Jul 2;15:1392483. doi: 10.3389/fphys.2024.1392483. eCollection 2024. Front Physiol. 2024. PMID: 39015223 Free PMC article.
-
Differences in Endothelial Activation and Dysfunction Induced by Antiphospholipid Antibodies Among Groups of Patients With Thrombotic, Refractory, and Non-refractory Antiphospholipid Syndrome.Front Physiol. 2021 Dec 2;12:764702. doi: 10.3389/fphys.2021.764702. eCollection 2021. Front Physiol. 2021. PMID: 34925061 Free PMC article.
-
Transmembrane Protein-184A Interacts with Syndecan-4 and Rab GTPases and Is Required to Maintain VE-Cadherin Levels.Cells. 2025 Jun 3;14(11):833. doi: 10.3390/cells14110833. Cells. 2025. PMID: 40498012 Free PMC article.
-
Role of Vascular Smooth Muscle Cell Phenotype Switching in Arteriogenesis.Int J Mol Sci. 2021 Sep 30;22(19):10585. doi: 10.3390/ijms221910585. Int J Mol Sci. 2021. PMID: 34638923 Free PMC article. Review.
References
-
- Arias-Díaz J, Vara E, Torres-Melero J, García C, Hernández J, Balibrea JL. Local production of oxygen free radicals and nitric oxide in rat diaphragm during sepsis: effects of pentoxifylline and somatostatin. Eur J Surg 163: 619–625, 1997. - PubMed
-
- Arredondo Zamarripa D, Noguez Imm R, Bautista Cortés AM, Vázquez Ruíz O, Bernardini M, Fiorio Pla A, Gkika D, Prevarskaya N, López-Casillas F, Liedtke W, Clapp C, Thébault S. Dual contribution of TRPV4 antagonism in the regulatory effect of vasoinhibins on blood-retinal barrier permeability: diabetic milieu makes a difference. Sci Rep 7: 13094, 2017. [Erratum in Sci Rep 8: 9652, 2018.] doi:10.1038/s41598-017-13621-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

