Hypofibrinolysis induced by tranexamic acid does not influence inflammation and mortality in a polymicrobial sepsis model
- PMID: 31891611
- PMCID: PMC6938370
- DOI: 10.1371/journal.pone.0226871
Hypofibrinolysis induced by tranexamic acid does not influence inflammation and mortality in a polymicrobial sepsis model
Abstract
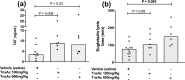
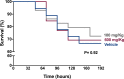
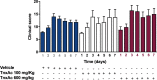
The biological relevance of fibrinolysis to the host response to sepsis is illustrated by pathogens such as S. pyogenes and Y. pestis, whose virulence factors are proteins that challenge the balance between pro- and anti-fibrinolytic factors of the host, and by the consistent finding of hypofibrinolysis in the early stages of sepsis. Whether this hypofibrinolytic response is beneficial or detrimental to the host, by containing the spread of pathogens while at the same time limiting the access of immune cell to infectious foci, is still a matter of debate. Tranexamic acid (TnxAc) is an antifibrinolytic agent that is being increasingly used to prevent and control bleeding in conditions such as elective orthopedic surgery, trauma, and post-partum-hemorrhage, which are frequently followed by infection and sepsis. Here we used a model of polymicrobial sepsis to evaluate whether hypofibrinolysis induced by TnxAc influenced survival, tissue injury and pathogen spread. Mice were treated with two doses of TnxAc bid for 48h, and then sepsis was induced by cecal ligation and puncture. Despite the induction of hypofibrinolysis by TnxAc, no difference could be observed in survival, tissue injury (measured by biochemical and histological parameters), cytokine levels or pathogen spread. Our results contribute with a new piece of data to the understanding of the complex interplay between fibrinolysis and innate immunity. While our results do not support the use of TnxAc in sepsis, they also address the thrombotic safety of TnxAc, a low cost and widely used agent to prevent bleeding.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Abrogating fibrinolysis does not improve bleeding or rFVIIa/rFVIII treatment in a non-mucosal venous injury model in haemophilic rodents.J Thromb Haemost. 2018 Jul;16(7):1369-1382. doi: 10.1111/jth.14148. Epub 2018 Jun 21. J Thromb Haemost. 2018. PMID: 29758126 Free PMC article.
-
Influence of Tranexamic Acid on Inflammatory Signaling in Trauma.Semin Thromb Hemost. 2020 Mar;46(2):183-188. doi: 10.1055/s-0040-1702169. Epub 2020 Mar 11. Semin Thromb Hemost. 2020. PMID: 32160643 Review.
-
Tranexamic acid modulates the cellular immune profile after traumatic brain injury in mice without hyperfibrinolysis.J Thromb Haemost. 2019 Dec;17(12):2174-2187. doi: 10.1111/jth.14603. Epub 2019 Sep 3. J Thromb Haemost. 2019. PMID: 31393041
-
Tranexamic Acid Influences the Immune Response, but not Bacterial Clearance in a Model of Post-Traumatic Brain Injury Pneumonia.J Neurotrauma. 2019 Dec 1;36(23):3297-3308. doi: 10.1089/neu.2018.6030. Epub 2019 Aug 2. J Neurotrauma. 2019. PMID: 31140372
-
Basis of antifibrinolytic therapy.J Clin Pathol Suppl (R Coll Pathol). 1980;14:35-40. J Clin Pathol Suppl (R Coll Pathol). 1980. PMID: 6159375 Free PMC article. Review. No abstract available.
References
-
- Bergmann S, Hammerschmidt S. Fibrinolysis and host response in bacterial infections. Thromb Haemost. 2007;98: 512–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

