A modern reassessment of glycoprotein-specific direct platelet autoantibody testing in immune thrombocytopenia
- PMID: 31891657
- PMCID: PMC6960466
- DOI: 10.1182/bloodadvances.2019000868
A modern reassessment of glycoprotein-specific direct platelet autoantibody testing in immune thrombocytopenia
Abstract
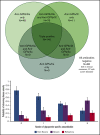
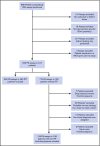
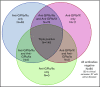
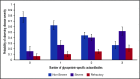
Platelet autoantibody (PA) testing has previously shown poor sensitivity for immune thrombocytopenia (ITP) diagnosis, but no previous study used both 2011 American Society of Hematology (ASH) guidelines for ITP diagnosis and 2012 International Society on Thrombosis and Haemostasis (ISTH) PA testing recommendations. We therefore performed a comprehensive retrospective study of PA testing in adult patients with ITP strictly applying these criteria. Of 986 PA assays performed, 485 assays in 368 patients met criteria and were included. Sensitivity and specificity of a positive test result for diagnosis of active ITP (n = 228 patients) were 90% and 78%, respectively. Sensitivity and specificity of a negative test result for clinical remission (n = 61 assays) were 87% and 91%. Antibodies against both glycoprotein IIb (GPIIb)/IIIa and GPIb/IX were required for the presence of antibodies against GPIa/IIa in patients with ITP. Logistic regression analysis revealed that more positive autoantibodies predicted more severe disease (relative to nonsevere ITP, relative risk ratio for severe ITP and refractory ITP was 2.27 [P < .001] and 3.09 [P < .001], respectively, per additional autoantibody); however, serologic testing did not meaningfully predict treatment response to glucocorticoids, intravenous immunoglobulin, or thrombopoietin receptor agonists. Sixty-four patients with ITP had multiple PA assays performed longitudinally: all 10 patients achieving remission converted from positive to negative serologic results, and evidence for epitope spreading was observed in 35% of patients with ongoing active disease. In conclusion, glycoprotein-specific direct PA testing performed using ISTH recommendations in patients meeting ASH diagnostic criteria is sensitive and specific for ITP diagnosis and reliably confirms clinical remission. More glycoproteins targeted by autoantibodies predicts for more severe disease.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: H.A.-S. reports consultancies with Agios, Dova, and Moderna; and research funding from Agios and Dova. D.J.K. reports research funding from Protalex, Bristol-Myers Squibb, Rigel, Bioverativ, Agios, Syntimmune, Principia, and Alnylam; and consultancies with ONO, Pfizer, 3SBios, Eisai, GlaxoSmithKline, Genzyme, Shire, Amgen, Shionogi, Rigel, Syntimmune, MedImmune, Novartis, Alexion, Bioverativ, argenx, Zafgen, Fujifilm, Principia, Kyowa Kirin, Takeda, and Platelet Disorders Support Group. The remaining authors declare no competing financial interests.
Figures
Similar articles
-
Association of autoantibody specificity and response to intravenous immunoglobulin G therapy in immune thrombocytopenia: a multicenter cohort study.J Thromb Haemost. 2014 Apr;12(4):497-504. doi: 10.1111/jth.12524. J Thromb Haemost. 2014. PMID: 24517219 Clinical Trial.
-
[Relationship between the expression of autoantibodies against platelet membrane glycoprotein and therapeutic effect in primary immune thrombocytopenia].Zhonghua Xue Ye Xue Za Zhi. 2013 Jul;34(7):610-3. doi: 10.3760/cma.j.issn.0253-2727.2013.07.011. Zhonghua Xue Ye Xue Za Zhi. 2013. PMID: 23906456 Chinese.
-
Anti-GPIb/IX autoantibodies are associated with poor response to dexamethasone combined with rituximab therapy in primary immune thrombocytopenia patients.Platelets. 2023 Dec;34(1):2258988. doi: 10.1080/09537104.2023.2258988. Epub 2023 Sep 18. Platelets. 2023. PMID: 37722393
-
The sensitivity and specificity of platelet autoantibody testing in immune thrombocytopenia: a systematic review and meta-analysis of a diagnostic test.J Thromb Haemost. 2019 May;17(5):787-794. doi: 10.1111/jth.14419. Epub 2019 Mar 20. J Thromb Haemost. 2019. PMID: 30801909
-
Anti-platelet antibody immunoassays in childhood immune thrombocytopenia: a systematic review.Vox Sang. 2020 May;115(4):323-333. doi: 10.1111/vox.12894. Epub 2020 Feb 20. Vox Sang. 2020. PMID: 32080872 Free PMC article.
Cited by
-
The common variable immunodeficiency IgM repertoire narrowly recognizes erythrocyte and platelet glycans.J Allergy Clin Immunol. 2024 Sep;154(3):778-791.e9. doi: 10.1016/j.jaci.2024.04.018. Epub 2024 Apr 29. J Allergy Clin Immunol. 2024. PMID: 38692308
-
How we treat primary immune thrombocytopenia in adults.J Hematol Oncol. 2023 Jan 19;16(1):4. doi: 10.1186/s13045-023-01401-z. J Hematol Oncol. 2023. PMID: 36658588 Free PMC article. Review.
-
Biological stratification of clinical disease courses in childhood immune thrombocytopenia.J Thromb Haemost. 2021 Apr;19(4):1071-1081. doi: 10.1111/jth.15232. Epub 2021 Mar 18. J Thromb Haemost. 2021. PMID: 33386662 Free PMC article.
-
Rilzabrutinib for the Treatment of Immune Thrombocytopenia.Eur J Haematol. 2025 Jul;115(1):4-15. doi: 10.1111/ejh.14425. Epub 2025 Apr 13. Eur J Haematol. 2025. PMID: 40222822 Free PMC article. Review.
-
Proteomic-Based Discovery of Predictive Biomarkers for Drug Therapy Response and Personalized Medicine in Chronic Immune Thrombocytopenia.Biomed Res Int. 2023 Oct 31;2023:9573863. doi: 10.1155/2023/9573863. eCollection 2023. Biomed Res Int. 2023. PMID: 37942029 Free PMC article.
References
-
- Harrington WJ, Sprague CC, Minnich V, Moore CV, Aulvin RC, Dubach R. Immunologic mechanisms in idiopathic and neonatal thrombocytopenic purpura. Ann Intern Med. 1953;38(3):433-469. - PubMed
-
- Shulman NR, Marder VJ, Weinrach RS. Similarities between known antiplatelet antibodies and the factor responsible for thrombocytopenia in idiopathic purpura. Physiologic, serologic and isotopic studies. Ann N Y Acad Sci. 1965;124(2):499-542. - PubMed
-
- Shulman NR, Weinrach RS, Libre EP, Andrews HL. The role of the reticuloendothelial system in the pathogenesis of idiopathic thrombocytopenic purpura. Trans Assoc Am Physicians. 1965;78:374-390. - PubMed
-
- Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr., Crowther MA; American Society of Hematology . The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190-4207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

