Progression of acute-to-chronic atopic dermatitis is associated with quantitative rather than qualitative changes in cytokine responses
- PMID: 31891686
- PMCID: PMC7214216
- DOI: 10.1016/j.jaci.2019.11.047
Progression of acute-to-chronic atopic dermatitis is associated with quantitative rather than qualitative changes in cytokine responses
Erratum in
-
Corrigendum.J Allergy Clin Immunol. 2023 May;151(5):1413. doi: 10.1016/j.jaci.2023.02.019. J Allergy Clin Immunol. 2023. PMID: 37149372 No abstract available.
Abstract
Background: Although multiple studies have assessed molecular changes in chronic atopic dermatitis (AD) lesions, little is known about the transition from acute to chronic disease stages, and the factors and mechanisms that shape chronic inflammatory activity.
Objectives: We sought to assess the global transcriptome changes that characterize the progression from acute to chronic stages of AD.
Methods: We analyzed transcriptome changes in paired nonlesional skin, acute and chronic AD lesions from 11 patients and 38 healthy controls by RNA-sequencing, and conducted in vivo and histological assays to evaluate findings.
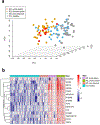
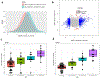
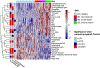
Results: Our data demonstrate that approximately 74% of the genes dysregulated in acute lesions remain or are further dysregulated in chronic lesions, whereas only 34% of the genes dysregulated in chronic lesions are altered already in the acute stage. Nonlesional AD skin exhibited enrichment of TNF, TH1, TH2, and TH17 response genes. Acute lesions showed marked dendritic-cell signatures and a prominent enrichment of TH1, TH2, and TH17 responses, along with increased IL-36 and thymic stromal lymphopoietin expression, which were further heightened in chronic lesions. In addition, genes involved in skin barrier repair, keratinocyte proliferation, wound healing, and negative regulation of T-cell activation showed a significant dysregulation in the chronic versus acute comparison. Furthermore, our data show progressive changes in vasculature and maturation of dendritic-cell subsets with chronicity, with FOXK1 acting as immune regulator.
Conclusions: Our results show that the changes accompanying the transition from nonlesional to acute to chronic inflammation in AD are quantitative rather than qualitative, with chronic AD having heightened TH2, TH1, TH17, and IL36 responses and skin barrier repair mechanisms. These findings provide novel insights and highlight underappreciated pathways in AD pathogenesis that may be amenable to therapeutic targeting.
Keywords: Atopic dermatitis; RNA-sequencing; acute AD; chronic AD; nonlesional.
Copyright © 2019 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest
All other authors declare no relevant conflicts of interest.
Figures

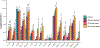
Comment in
-
Shifting paradigms in the immunology of atopic dermatitis.J Allergy Clin Immunol. 2020 May;145(5):1360-1362. doi: 10.1016/j.jaci.2020.02.030. Epub 2020 Mar 14. J Allergy Clin Immunol. 2020. PMID: 32179160 No abstract available.
References
-
- Lloyd-Lavery A, Solman L, Grindlay DJC, Rogers NK, Thomas KS, Harman KE. What’s new in atopic eczema? An analysis of systematic reviews published in 2016. Part 2: Epidemiology, aetiology and risk factors. Clin Exp Dermatol 2019; 44:370–5. - PubMed
-
- Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers 2018; 4:1. - PubMed
-
- Oyoshi MK, He R, Kumar L, Yoon J, Geha RS. Cellular and molecular mechanisms in atopic dermatitis. Adv Immunol 2009; 102:135–226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

