Flow-Based Three-Dimensional Co-Culture Model for Long-Term Hepatotoxicity Prediction
- PMID: 31892214
- PMCID: PMC7019533
- DOI: 10.3390/mi11010036
Flow-Based Three-Dimensional Co-Culture Model for Long-Term Hepatotoxicity Prediction
Abstract
We developed concave microwell arrays to establish a size-controllable 3-D co-culture liver model for in vitro drug toxicity testing, to predict hepatotoxicity. The interaction of hepatocytes with hepatic stellate cells (HSCs) was investigated by co-culturing primary 3-D hepatocyte spheroids and HSCs (heterosphere), using 3-D liver-on-a-chip. The effect of HSCs was investigated during spheroid formation; they were involved in controlling the organization of spheroidal aggregates and the formation of tight cell-cell contacts. Scanning electron microscopy (SEM) images showed that co-cultured spheroids with smoother surfaces in the flow chip aggregated more tightly and rapidly, compared to mono-cultured spheroids, until 13 days. Metabolic function analysis revealed that heterospheres secreted 40% more albumin and urea than hepatospheres on day 13. Additionally, an acetaminophen (AAP) and isoniazid (INH) concentration-dependent increase in CYP3A4 expression was detected in the 3-D cultures, and an increase in Lactate dehydrogenase (LDH) release after AAP and INH treatment was observed. CYP1A2, Mrp1 and UGT1A5 mRNA expression levels in the heterospheres and hepatospheres were evaluated from days 3 to 13. To examine the potential for toxicity testing in the flow-conditioned culture of the heterospheres, we evaluated cytotoxicity using the endpoint LDH release in the heterospheres and hepatospheres. IC50 values for AAP and INH after 24 h of exposure were calculated from the dose-response curves of the compounds. Flow-conditioned heterosphere culture results suggest that it may be suitable for long-term culture and cytotoxicity testing. Thus, our co-culture system closely resembles the in vivo environment and allows long-term in vitro hepatotoxicity prediction.
Keywords: co-culture; hepatic stellate cell; hepatotoxicity; microfluidic; spheroid.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures




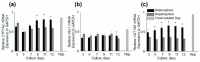

Similar articles
-
Concave microwell based size-controllable hepatosphere as a three-dimensional liver tissue model.Biomaterials. 2011 Nov;32(32):8087-96. doi: 10.1016/j.biomaterials.2011.07.028. Epub 2011 Aug 2. Biomaterials. 2011. PMID: 21813175
-
Spheroid-based three-dimensional liver-on-a-chip to investigate hepatocyte-hepatic stellate cell interactions and flow effects.Lab Chip. 2013 Sep 21;13(18):3529-37. doi: 10.1039/c3lc50197c. Lab Chip. 2013. PMID: 23657720
-
3D co-culturing model of primary pancreatic islets and hepatocytes in hybrid spheroid to overcome pancreatic cell shortage.Biomaterials. 2013 May;34(15):3784-94. doi: 10.1016/j.biomaterials.2013.02.010. Epub 2013 Feb 21. Biomaterials. 2013. PMID: 23433671
-
Sandwich-cultured hepatocytes: utility for in vitro exploration of hepatobiliary drug disposition and drug-induced hepatotoxicity.Expert Opin Drug Metab Toxicol. 2013 May;9(5):589-616. doi: 10.1517/17425255.2013.773973. Epub 2013 Mar 2. Expert Opin Drug Metab Toxicol. 2013. PMID: 23452081 Review.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
Cited by
-
Liver microphysiological platforms for drug metabolism applications.Cell Prolif. 2021 Sep;54(9):e13099. doi: 10.1111/cpr.13099. Epub 2021 Jul 22. Cell Prolif. 2021. PMID: 34291515 Free PMC article. Review.
-
Three-Dimensional Liver Culture Systems to Maintain Primary Hepatic Properties for Toxicological Analysis In Vitro.Int J Mol Sci. 2021 Sep 23;22(19):10214. doi: 10.3390/ijms221910214. Int J Mol Sci. 2021. PMID: 34638555 Free PMC article. Review.
-
Microphysiological Models for Mechanistic-Based Prediction of Idiosyncratic DILI.Cells. 2023 May 25;12(11):1476. doi: 10.3390/cells12111476. Cells. 2023. PMID: 37296597 Free PMC article. Review.
-
Fabrication of Concave Microwells and Their Applications in Micro-Tissue Engineering: A Review.Micromachines (Basel). 2022 Sep 19;13(9):1555. doi: 10.3390/mi13091555. Micromachines (Basel). 2022. PMID: 36144178 Free PMC article. Review.
-
Recent Advances in Organ-on-Chips Integrated with Bioprinting Technologies for Drug Screening.Adv Healthc Mater. 2023 Aug;12(20):e2203172. doi: 10.1002/adhm.202203172. Epub 2023 May 14. Adv Healthc Mater. 2023. PMID: 36971091 Free PMC article. Review.
References
-
- Hewitt N.J., Lechón M.J., Houston J.B., Hallifax D., Brown H.S., Maurel P., Kenna J.G., Gustavsson L., Lohmann C., Skonberg C., et al. Primary hepatocytes: Current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab. Rev. 2007;39:159–234. doi: 10.1080/03602530601093489. - DOI - PubMed
-
- Jones C.N., Tuleuova N., Lee J.Y., Ramanculov E., Reddi A.H., Zern M.A., Revzin A. Cultivating hepatocytes on printed arrays of HGF and BMP7 to characterize protective effects of these growth factors during in vitro alcohol injury. Biomaterials. 2010;31:5936–5944. doi: 10.1016/j.biomaterials.2010.04.006. - DOI - PMC - PubMed
-
- Sivaraman A., Leach J.K., Townsend S., Iida T., Hogan B.J., Stolz D.B., Fry R., Samson L.D., Tannenbaum S.R., Griffith L.G. A microscale in vitro physiological model of the liver: Predictive screens for drug metabolism and enzyme induction. Curr. Drug. Metab. 2005;6:569–591. doi: 10.2174/138920005774832632. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

