N-(2-Arylmethylthio-4-Chloro-5-Methylbenzenesulfonyl)amide Derivatives as Potential Antimicrobial Agents-Synthesis and Biological Studies
- PMID: 31892248
- PMCID: PMC6981581
- DOI: 10.3390/ijms21010210
N-(2-Arylmethylthio-4-Chloro-5-Methylbenzenesulfonyl)amide Derivatives as Potential Antimicrobial Agents-Synthesis and Biological Studies
Abstract
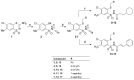
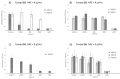
Rising resistance of pathogenic bacteria reduces the options of treating hospital and non-hospital bacterial infections. There is a need to search for newer chemotherapies that will show antimicrobial ability against planktonic cells as well as bacterial biofilms. We have synthesized a series of N-(2-arylmethylthio-4-chloro-5-methylbenzenesulfonyl)amides, namely, molecular hybrids, which include a 2-mercaptobenzenosulfonamide fragment and either cinnamic or cyclohexylpropionic acid residues. The antimicrobial activity of compounds 8‒17 was evaluated on Gram-positive, Gram-negative bacteria and fungal species. Experiments took into account investigation of antibacterial activity against planktonic cells as well as biofilms. Compounds 8‒17 showed high bacteriostatic activity against staphylococci, with the most active molecules 10 and 16 presenting low MIC values of 4-8 μg/mL against reference methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-sensitive S. aureus (MSSA) strains as well as clinical isolates. Compounds 10 and 16 also showed an ability to inhibit biofilms formed by MRSA and MSSA. The potential of 10 and 16 as antibiofilm agents was supported by cytotoxicity assays that indicated no cytotoxic effect either on normal cells of human keratinocytes or on human cancer cells, including cervical, colon, and breast cancer lines.
Keywords: MRSA; antibacterial; benzenesulfonamide; biofilm; cytotoxicity; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- World Health Organization (WHO) Fact Sheet: Antimicrobial Resistance. [(accessed on 27 November 2019)]; Available online: http://www.who.int/mediacentre/factsheets/fs194/en/
-
- Cantas L., Shah S.Q., Cavaco L.M., Manaia C.M., Walsh F., Popowska M., Garelick H., Bürgmann H., Sørum H. A brief multi-disciplinary review on antimicrobial resistance in medicine and its linkage to the global environmental microbiota. Front. Microbiol. 2013;4:96. doi: 10.3389/fmicb.2013.00096. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

