Angiogenic Potential of Bone Marrow Derived CD133+ and CD271+ Intramyocardial Stem Cell Trans- Plantation Post MI
- PMID: 31892273
- PMCID: PMC7016579
- DOI: 10.3390/cells9010078
Angiogenic Potential of Bone Marrow Derived CD133+ and CD271+ Intramyocardial Stem Cell Trans- Plantation Post MI
Abstract
Background: Bone marrow (BM)-derived stem cells with their various functions and characteristics have become a well-recognized source for the cell-based therapies. However, knowledge on their therapeutic potential and the shortage for a cross-link between distinct BM-derived stem cells, primed after the onset of myocardial infarction (MI), seems to be still rudimentary. Therefore, the post-examination of the therapeutic characteristics of such primed hematopoietic CD133+ and mesenchymal CD271+ stem cells was the object of the present study.
Methods and results: The effects of respective CD133+ and CD271+ mononuclear cells alone as well as in the co-culture model have been explored with focus on their angiogenic potential. The phenotypic analysis revealed a small percentage of isolated cells expressing both surface markers. Moreover, target stem cells isolated with our standardized immunomagnetic isolation procedure did not show any negative alterations following BM storage in regard to cell numbers and/or quality. In vitro network formation relied predominantly on CD271+ stem cells when compared with single CD133+ culture. Interestingly, CD133+ cells contributed in the tube formation, only if they were cultivated in combination with CD271+ cells. Additional to the in vitro examination, therapeutic effects of the primed stem cells were investigated 48 h post MI in a murine model. Hence, we have found a lower expression of transforming growth factor βeta 3 (TGFβ3) as well as an increase of the proangiogenic factors after CD133+ cell treatment in contrast to CD271+ cell treatment. On the other hand, the CD271+ cell therapy led to a lower expression of the inflammatory cytokines.
Conclusion: The interactions between CD271+ and CD133+ subpopulations the extent to which the combination may enhance cardiac regeneration has still not been investigated so far. We expect that the multiple characteristics and various regenerative effects of CD271+ cells alone as well as in combination with CD133+ will result in an improved therapeutic impact on ischemic heart disease.
Keywords: angiogenesis; bone marrow stem cells; cardiac regeneration; myocardial infarction.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

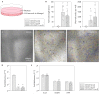

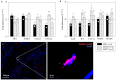
References
-
- Sánchez-Guijo F., Caballero-Velázquez T., López-Villar O., Redondo A., Parody R., Martinez C., Olavarria E., Andreu E., Prosper F., Diez-Campelo M., et al. Sequential Third-Party Mesenchymal Stromal Cell Therapy for Refractory Acute Graft-Versus-Host Disease. Biol. Blood Marrow Transplant. 2014;20:1580–1585. doi: 10.1016/j.bbmt.2014.06.015. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

