Disrupted Calcium Signaling in Animal Models of Human Spinocerebellar Ataxia (SCA)
- PMID: 31892274
- PMCID: PMC6981692
- DOI: 10.3390/ijms21010216
Disrupted Calcium Signaling in Animal Models of Human Spinocerebellar Ataxia (SCA)
Abstract
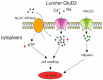
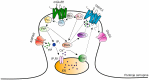
Spinocerebellar ataxias (SCAs) constitute a heterogeneous group of more than 40 autosomal-dominant genetic and neurodegenerative diseases characterized by loss of balance and motor coordination due to dysfunction of the cerebellum and its efferent connections. Despite a well-described clinical and pathological phenotype, the molecular and cellular events that underlie neurodegeneration are still poorly undaerstood. Emerging research suggests that mutations in SCA genes cause disruptions in multiple cellular pathways but the characteristic SCA pathogenesis does not begin until calcium signaling pathways are disrupted in cerebellar Purkinje cells. Ca2+ signaling in Purkinje cells is important for normal cellular function as these neurons express a variety of Ca2+ channels, Ca2+-dependent kinases and phosphatases, and Ca2+-binding proteins to tightly maintain Ca2+ homeostasis and regulate physiological Ca2+-dependent processes. Abnormal Ca2+ levels can activate toxic cascades leading to characteristic death of Purkinje cells, cerebellar atrophy, and ataxia that occur in many SCAs. The output of the cerebellar cortex is conveyed to the deep cerebellar nuclei (DCN) by Purkinje cells via inhibitory signals; thus, Purkinje cell dysfunction or degeneration would partially or completely impair the cerebellar output in SCAs. In the absence of the inhibitory signal emanating from Purkinje cells, DCN will become more excitable, thereby affecting the motor areas receiving DCN input and resulting in uncoordinated movements. An outstanding advantage in studying the pathogenesis of SCAs is represented by the availability of a large number of animal models which mimic the phenotype observed in humans. By mainly focusing on mouse models displaying mutations or deletions in genes which encode for Ca2+ signaling-related proteins, in this review we will discuss the several pathogenic mechanisms related to deranged Ca2+ homeostasis that leads to significant Purkinje cell degeneration and dysfunction.
Keywords: Ca2+ signaling; Purkinje cells; spinocerebellar ataxias.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

