The Biosurfactant β-Aescin: A Review on the Physico-Chemical Properties and Its Interaction with Lipid Model Membranes and Langmuir Monolayers
- PMID: 31892278
- PMCID: PMC6983251
- DOI: 10.3390/molecules25010117
The Biosurfactant β-Aescin: A Review on the Physico-Chemical Properties and Its Interaction with Lipid Model Membranes and Langmuir Monolayers
Abstract
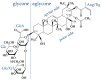
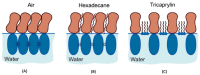
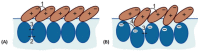
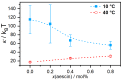
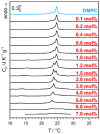
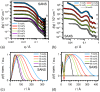
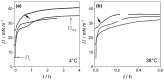
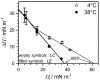
This review discusses recent progress in physicochemical understanding of the action of the saponin β -aescin (also called β -escin), the biologically active component in the seeds of the horse chestnut tree Aesculus hippocastanum. β -Aescin is used in pharmacological and cosmetic applications showing strong surface activity. In this review, we outline the most important findings describing the behavior of β -aescin in solution (e.g., critical micelle concentration ( c m c ) and micelle shape) and special physicochemical properties of adsorbed β -aescin monolayers at the air-water and oil-water interface. Such monolayers were found to posses very special viscoelastic properties. The presentation of the experimental findings is complemented by discussing recent molecular dynamics simulations. These simulations do not only quantify the predominant interactions in adsorbed monolayers but also highlight the different behavior of neutral and ionized β -aescin molecules. The review concludes on the interaction of β -aescin with phospholipid model membranes in the form of bilayers and Langmuir monolayers. The interaction of β -aescin with lipid bilayers was found to strongly depend on its c m c . At concentrations below the c m c , membrane parameters are modified whereas above the c m c , complete solubilization of the bilayers occurs, depending on lipid phase state and concentration. In the presence of gel-phase phospholipids, discoidal bicelles form; these are tunable in size by composition. The phase behavior of β -aescin with lipid membranes can also be modified by addition of other molecules such as cholesterol or drug molecules. The lipid phase state also determines the penetration rate of β -aescin molecules into lipid monolayers. The strongest interaction was always found in the presence of gel-phase phospholipid molecules.
Keywords: Aesculus hippocastanum; Langmuir monolayers; air–water interface, MD simulation; critical micelle concentration cmc; lipid membrane; saponin; β-aescin; β-escin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Hostettmann K., Marston A. Saponins. Cambridge University Press; Cambridge, UK: New York, NY, USA: 1995. - DOI
-
- Cheok C.Y., Salman H.A.K., Sulaiman R. Extraction and quantification of saponins: A review. Food Res. Int. 2014;59:16–40. doi: 10.1016/j.foodres.2014.01.057. - DOI
-
- Netala V.R., Ghosh S.B., Bobbu P., Anitha D., Tartte V. Triterpenoid saponins: A review on biosynthesis, applications and mechanism of their action. Int. Pharm. Pharm. Sci. 2015;7:24-8.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

