Craniosacral therapy for chronic pain: a systematic review and meta-analysis of randomized controlled trials
- PMID: 31892357
- PMCID: PMC6937867
- DOI: 10.1186/s12891-019-3017-y
Craniosacral therapy for chronic pain: a systematic review and meta-analysis of randomized controlled trials
Abstract
Objectives: To systematically assess the evidence of Craniosacral Therapy (CST) for the treatment of chronic pain.
Methods: PubMed, Central, Scopus, PsycInfo and Cinahl were searched up to August 2018. Randomized controlled trials (RCTs) assessing the effects of CST in chronic pain patients were eligible. Standardized mean differences (SMD) and 95% confidence intervals (CI) were calculated for pain intensity and functional disability (primary outcomes) using Hedges' correction for small samples. Secondary outcomes included physical/mental quality of life, global improvement, and safety. Risk of bias was assessed using the Cochrane tool.
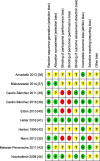
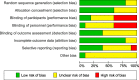
Results: Ten RCTs of 681 patients with neck and back pain, migraine, headache, fibromyalgia, epicondylitis, and pelvic girdle pain were included. CST showed greater post intervention effects on: pain intensity (SMD = -0.32, 95%CI = [- 0.61,-0.02]) and disability (SMD = -0.58, 95%CI = [- 0.92,-0.24]) compared to treatment as usual; on pain intensity (SMD = -0.63, 95%CI = [- 0.90,-0.37]) and disability (SMD = -0.54, 95%CI = [- 0.81,-0.28]) compared to manual/non-manual sham; and on pain intensity (SMD = -0.53, 95%CI = [- 0.89,-0.16]) and disability (SMD = -0.58, 95%CI = [- 0.95,-0.21]) compared to active manual treatments. At six months, CST showed greater effects on pain intensity (SMD = -0.59, 95%CI = [- 0.99,-0.19]) and disability (SMD = -0.53, 95%CI = [- 0.87,-0.19]) versus sham. Secondary outcomes were all significantly more improved in CST patients than in other groups, except for six-month mental quality of life versus sham. Sensitivity analyses revealed robust effects of CST against most risk of bias domains. Five of the 10 RCTs reported safety data. No serious adverse events occurred. Minor adverse events were equally distributed between the groups.
Discussion: In patients with chronic pain, this meta-analysis suggests significant and robust effects of CST on pain and function lasting up to six months. More RCTs strictly following CONSORT are needed to further corroborate the effects and safety of CST on chronic pain.
Protocol registration at prospero: CRD42018111975.
Keywords: Chronic pain; Complementary therapies; Craniosacral therapy; Meta-analysis; Systematic review.
Conflict of interest statement
The authors declare no competing interests. The authors have no financial or non-financial association that might create a conflict of interest regarding the submitted manuscript.
Figures





References
-
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators: Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388(10053):1545–602. 10.1016/s0140-6736(16)31678-6. - PMC - PubMed
-
- Chou R, Deyo R, Friedly J, Skelly A, Weimer M, Fu R, Dana T, Kraegel P, Griffin J, Grusing S: Systemic pharmacologic therapies for low Back pain: a systematic review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2017;166(7):480–92. 10.7326/m16-2458. - PubMed
-
- Chenot JF, Becker A, Leonhardt C, Keller S, Donner-Banzhoff N, Baum E, Pfingsten M, Hildebrandt J, Basler HD, Kochen MM: Use of complementary alternative medicine for low back pain consulting in general practice: a cohort study. BMC Complement Altern Med. 2007;7:42. 10.1186/1472-6882-7-42. - PMC - PubMed
-
- Wolsko PM, Eisenberg DM, Davis RB, Kessler R, Phillips RS: Patterns and perceptions of care for treatment of back and neck pain: results of a national survey. Spine (Phila Pa 1976). 2003;28(3):292–8. 10.1097/01.Brs.0000042225.88095.7c. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

